Which word equation represents a neutralization reaction? Hcl + naoh h2o + nacl c.
Which Word Equation Represents A Neutralization Reaction. A salt is a neutral ionic compound. The reactants are potassium hydroxide and sulfuric acid. Let’s see how a neutralization reaction produces both water and a salt, using as an example the reaction between solutions of hydrochloric acid and sodium hydroxide. H2so4 + 2naoh → na2so4 + 2h2o.

- Which Equation Represents A Neutralization Reaction From studylib.net
Related Post 1. Which Equation Represents A Neutralization Reaction :
Predict the salt produced from the following neutralization reaction: What are the products of a reaction between koh(aq) and i hc1(aq)? A salt is a neutral ionic compound. A base + acid salt + water base + salt water + acid c) salt + acid base + water d) salt + water acid + base hgh 9.
Which equation represents a neutralization reaction?
Which word equation represents a neutralization reaction? Neutralisation reactions can be described using chemical equations like a word equation. The word equation for neutralization is acid + base = salt + water. Acid + salt → base + water. How many grams of caco3 are needed to neutralize 50 ml of stomach acid at ph = 2.0 completely, if the following equation represents the neutralization reaction? Neutralization reaction is defined as the reaction in which an acid reacts with a base to produce a salt and water molecule.

The chemical equation that represents neutralization reaction is. This uses the scientific names for the acid and alkali placed on the reactant side of the equation. What is the word equation for neutralization in chemistry?
 Source: transtutors.com
Source: transtutors.com
The equation for complete oxidation of glucose in anaerobic respiration is glucose > The chemical equation that represents neutralization reaction is. (1) base + acid => salt + water (2) base +salt => water + acid (3) salt + acid =>base + water
 Source: slideplayer.com
Source: slideplayer.com
H2so4 + 2naoh → na2so4 + 2h2o. A) base acid salt+ water b) base saltwater +acid c) salt +acid base + water d) salt +water acid + base 2. Which word equation represents a neutralization reaction?
 Source: youtube.com
Source: youtube.com
Ba(c 2 h 3 o We can write the overall equation for the reaction first. Acid + water → salt + base.
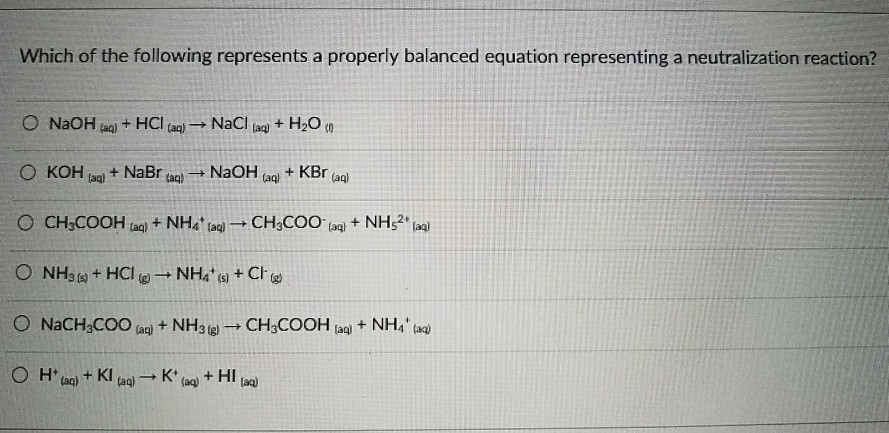
The chemical equation that represents neutralization reaction is. Acids and bases react with each other to produce water and ions. Neutralization is the reaction of an acid and a base, which forms water and a salt.
 Source: studylib.net
Source: studylib.net
The acid neutralizes the base, and hence, this reaction is called a neutralization reaction. Neutralisation reactions can be described using chemical equations like a word equation. Neutralization is the reaction of an acid and a base, which forms water and a salt.
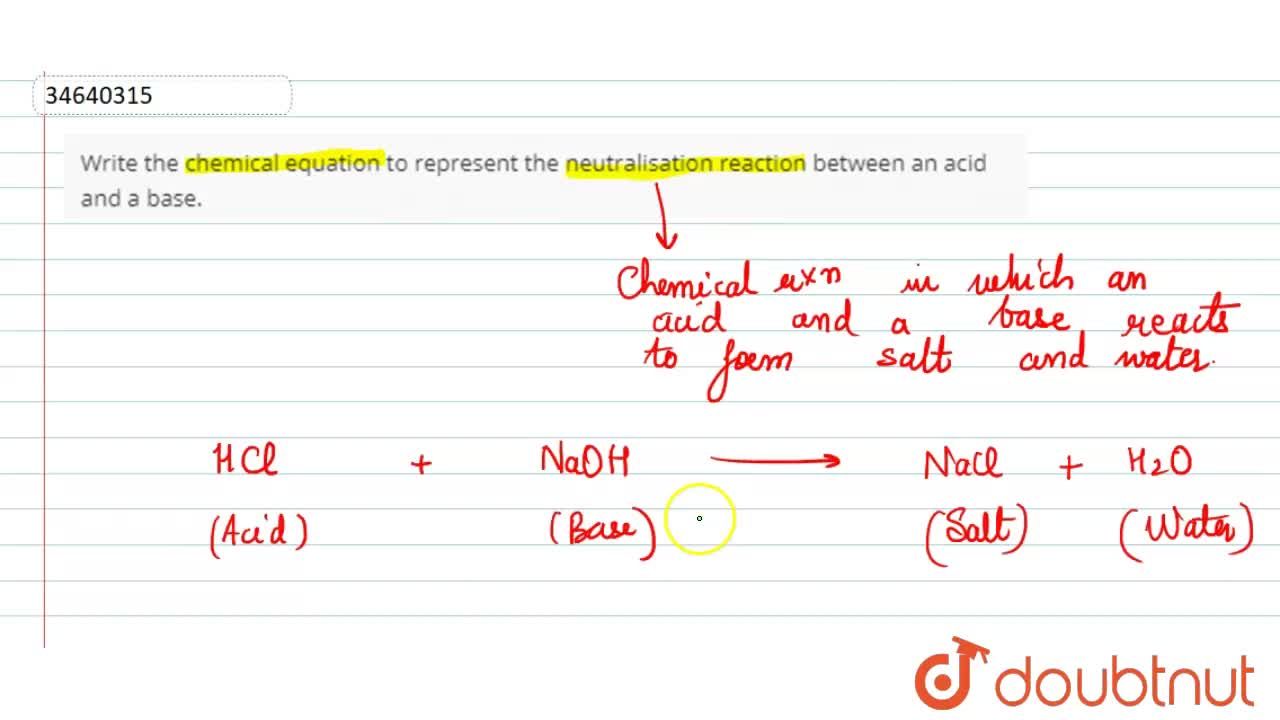 Source: doubtnut.com
Source: doubtnut.com
This is the reaction that always occurs when an acid + alkali salt + water. Ba(c 2 h 3 o (1) molecular formulas (2) structural formulas (3) total number of atoms per molecule (4) total number of bonds per molecule:
 Source: slidetodoc.com
Source: slidetodoc.com
This leaves the ionic equation. Which word equation represents a neutralization reaction? Let’s see how a neutralization reaction produces both water and a salt, using as an example the reaction between solutions of hydrochloric acid and sodium hydroxide.
 Source: yumpu.com
Source: yumpu.com
Discusses chemical reactions that occur when acids and bases interact. We can write the overall equation for the reaction first. Predict the salt produced from the following neutralization reaction:
 Source: oneclass.com
Source: oneclass.com
Which word equation represents a neutralization reaction? This uses the scientific names for the acid and alkali placed on the reactant side of the equation. The overall equation for this reaction is:

This uses the scientific names for the acid and alkali placed on the reactant side of the equation. (1) molecular formulas (2) structural formulas (3) total number of atoms per molecule (4) total number of bonds per molecule: The ph value of neutralisation is ph7 because ph1 is a strong acid, ph14 is a strong alkali.
 Source: studylib.net
Source: studylib.net
Learn about the net ionic equation, review examples of. However i to am trying to find the word equation as i have a test tomorrow that i. Neutralisation reactions can be described using chemical equations like a word equation.
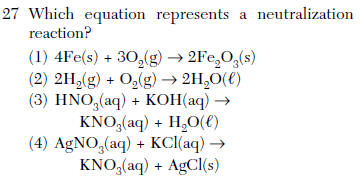 Source: kentchemistry.com
Source: kentchemistry.com
The arrhenius definition of a base is a substance that increases the amount of oh − in an aqueous solution. Potassium hydroxide + sulfuric acid → potassium sulfate + water. Which equation represents a neutralization reaction?
 Source: study.com
Source: study.com
Neutralization is a chemical reaction through which water and salt are formed as the result of a strong acid and strong base coming together. This is the reaction that always occurs when an acid + alkali salt + water. It is called the ionic equation for neutralisation.
 Source: studylib.net
Source: studylib.net
The arrhenius definition of a base is a substance that increases the amount of oh − in an aqueous solution. Based on the results of testing colorless solutions with indicators, which solution is most acidic? Potassium hydroxide + sulfuric acid → potassium sulfate + water.
 Source: chegg.com
Source: chegg.com
The word equation for neutralization is acid + base = salt + water. The acid neutralizes the base, and hence, this reaction is called a neutralization reaction. Let’s see how a neutralization reaction produces both water and a salt, using as an example the reaction between solutions of hydrochloric acid and sodium hydroxide.
 Source: yumpu.com
Source: yumpu.com
(1) molecular formulas (2) structural formulas (3) total number of atoms per molecule (4) total number of bonds per molecule: Net ionic equations for neutralization reactions may include solid acids, solid bases, solid salts, and water. Which of the following chemical reactions represents a neutralization reaction?
 Source: slideplayer.com
Source: slideplayer.com
Acid + salt → base + water. How many grams of caco3 are needed to neutralize 50 ml of stomach acid at ph = 2.0 completely, if the following equation represents the neutralization reaction? Neutralization reaction is defined as the reaction in which an acid reacts with a base to produce a salt and water molecule.
 Source: brainly.in
Source: brainly.in
Let’s see how a neutralization reaction produces both water and a salt, using as an example the reaction between solutions of hydrochloric acid and sodium hydroxide. A) base acid salt+ water b) base saltwater +acid c) salt +acid base + water d) salt +water acid + base 2. Acid + water → salt + base.
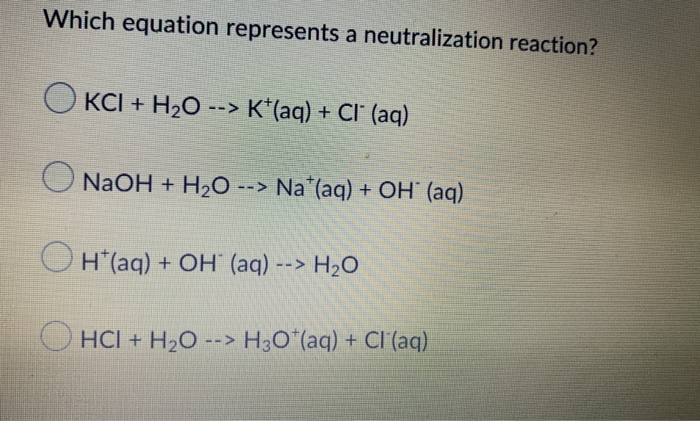 Source: chegg.com
Source: chegg.com
In plants end product alcohol, in animals it is lactate. Learn about the net ionic equation, review examples of. Let’s see how a neutralization reaction produces both water and a salt, using as an example the reaction between solutions of hydrochloric acid and sodium hydroxide.
Also Read :