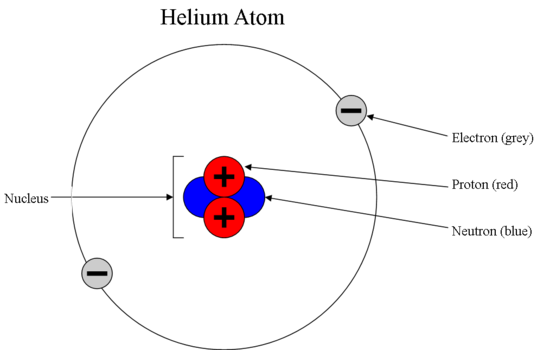
An atom has hardly any empty space, and the nucleus has a negative charge. There are two main ways a nucleus can form:
Which Subatomic Particles Are In The Nucleus. Subatomic particles can usually pass undeflected through an atom because the volume of an atom is composed of a. Nucleus has a positive charge. Which of these subatomic particles is found in the nucleus of an atom? The nuclei of all atoms contain subatomic particles called protons.
 Sub-Atomic Particles - Chemistry Libretexts From chem.libretexts.org
Sub-Atomic Particles - Chemistry Libretexts From chem.libretexts.org
Related Post Sub-Atomic Particles - Chemistry Libretexts :
Through nuclear fusion or nuclear fission. Click to see full answer. The three main subatomic particles that form an atom are protons, neutrons, and electrons. A subatomic particle is a unit of matter or energy that�s the fundamental makeup of all matter.
Electrons only if an atom has six positively charged subatomic particles, which of the following must it also have in order to become a neutral atom?
Nucleus has a positive charge. A third type of subatomic particle, electrons, move around the nucleus. There are three main types of subatomic particles in an atom, protons, neutrons, and electrons. The nuclei of most atoms also contain neutrons. The three main subatomic particles that form an atom are protons, neutrons, and electrons. There are three main subatomic particles.
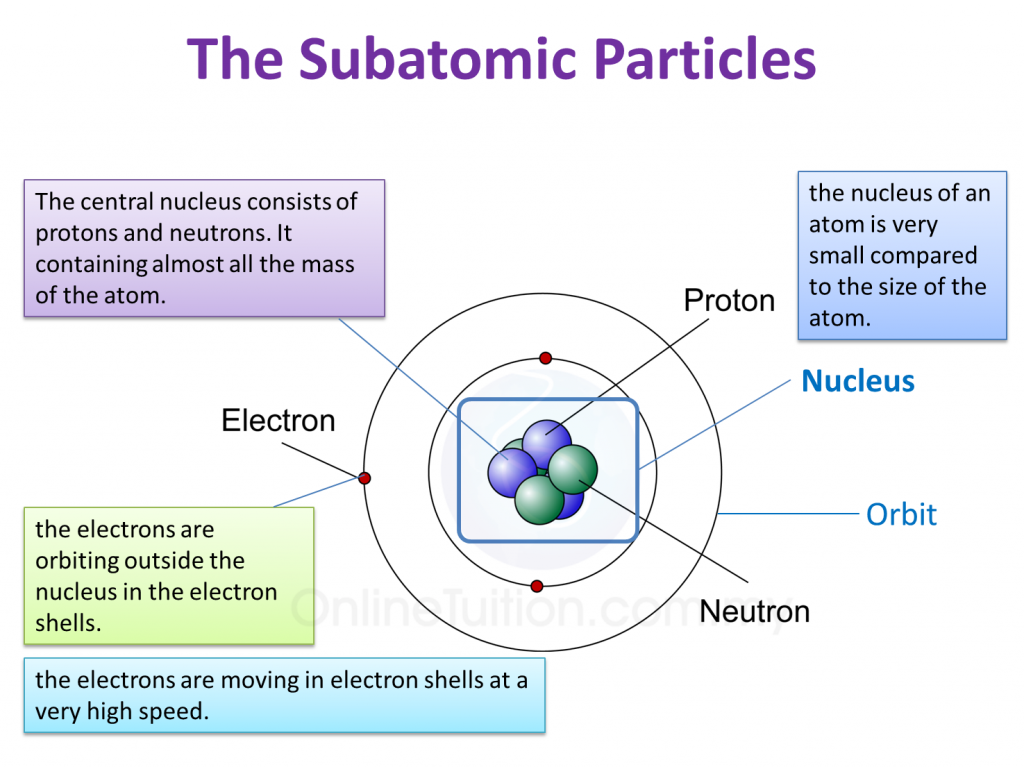 Source: content.myhometuition.com
Source: content.myhometuition.com
Protons and neutrons have the most mass. Nuclear physics deals with how protons and neutrons arrange themselves in nuclei. There are two main ways a nucleus can form:
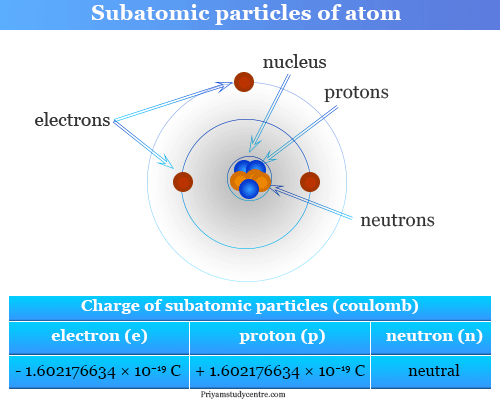 Source: priyamstudycentre.com
Source: priyamstudycentre.com
Electrons only if an atom has six positively charged subatomic particles, which of the following must it also have in order to become a neutral atom? Enter your answer as integers separated by commas. The nucleus of an atom contains which subatomic particles?
 Source: le.ac.uk
Source: le.ac.uk
It is possible for the nucleus of an atom to contain subatomic particles such as neutrons and protons in addition to electrons. Which of these subatomic particles is found in the nucleus of an atom? The nuclei of most atoms also contain neutrons.
 Source: slideplayer.com
Source: slideplayer.com
A third type of subatomic particle, electrons, move around the nucleus. The nucleus contains subatomic particles: Electrons are called to be the negatively charged subatomic particles
 Source: slideserve.com
Source: slideserve.com
The centre of the atom is called the nuclei; The study of subatomic particles, atoms and molecules, and their structure and interactions, requires quantum mechanics. According to modern atomic theory, an atom has a nucleus, which is its center, or core.
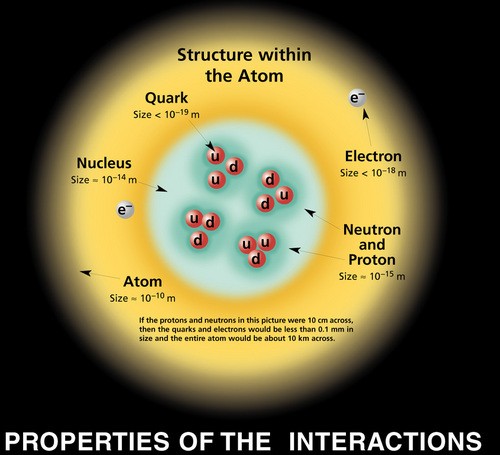 Source: prancer.physics.louisville.edu
Source: prancer.physics.louisville.edu
The nuclei of all atoms contain subatomic particles called protons. Nuclear physics deals with how protons and neutrons arrange themselves in nuclei. It has protons and neutrons.
 Source: slideplayer.com
Source: slideplayer.com
The center of the atom is called the nucleus. Subatomic particles include electrons, the negatively charged, almost massless particles that nevertheless account for most of the size of the atom, and they include the heavier building blocks of the small but very dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Subatomic particles can usually pass undeflected through an atom because the volume of an atom is composed of a.
 Source: youtube.com
Source: youtube.com
These electrons can be lost from or gained by an atom to form the ions. Some nuclei might also include other types of particle like pions or muons. There are three main subatomic particles.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Which particles are found in the nucleus of most atoms? These electrons can be lost from or gained by an atom to form the ions. The subatomic particles that are inside the nucleus are protons and neutrons.
 Source: brainly.com
Source: brainly.com
Electrons, which have a negative charge, are particles that can found orbiting outside the nucleus of. Both are together in the center of an atom, called the nucleus. There are three main types of subatomic particles in an atom, protons, neutrons, and electrons.
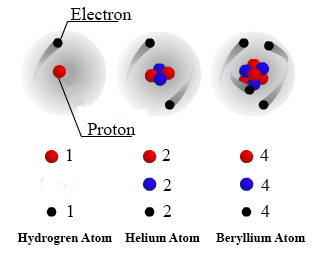 Source: nde-ed.org
Source: nde-ed.org
A third type of subatomic particle, electrons, move around the nucleus. The protons have a positive electrical charge and the neutrons have no electrical charge. It is a subatomic particle (symbol n or n 0), which has a neutral charge, and its mass is greater than a proton.
 Source: pngwing.com
Source: pngwing.com
Nucleus has a positive charge. James chadwick discovered that the nucleus had a new uncharged particle; It is a subatomic particle (symbol n or n 0), which has a neutral charge, and its mass is greater than a proton.
 Source: slideplayer.com
Source: slideplayer.com
The subatomic particles that are inside the nucleus are protons and neutrons. Enter your answer as integers separated by commas. The electron travels around out side the nucleus.
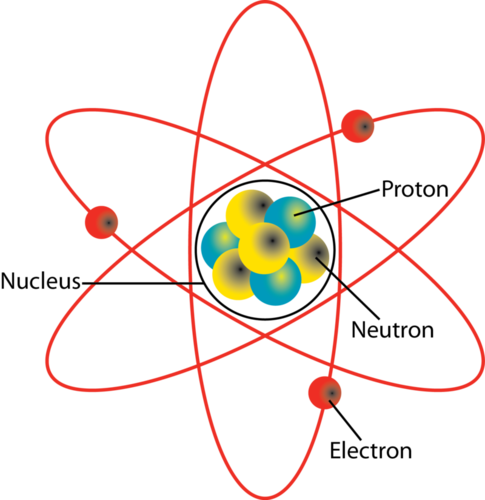 Source: socratic.org
Source: socratic.org
These two subatomic particles above are located in the nucleus. Electrons only if an atom has six positively charged subatomic particles, which of the following must it also have in order to become a neutral atom? Electrons are the subatomic particles which revolve around the nucleus of the atom.
 Source: study.com
Source: study.com
The electron travels around out side the nucleus. Which subatomic particle(s) would be found orbiting around the nucleus? The nuclei of most atoms also contain neutrons.
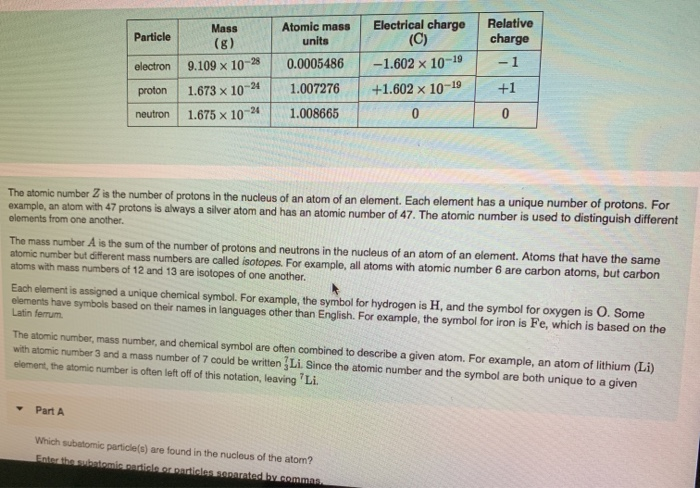 Source: chegg.com
Source: chegg.com
The protons and neutrons are in the nucleus at the center of the atom, whilst the electrons how many types of subatomic particles are there? These particles are collectively called nucleons). Subatomic particles can usually pass undeflected through an atom because the volume of an atom is composed of a.
 Source: flexiprep.com
Source: flexiprep.com
Some nuclei might also include other types of particle like pions or muons. Subatomic particles include electrons, the negatively charged, almost massless particles that nevertheless account for most of the size of the atom, and they include the heavier building blocks of the small but very dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. A subatomic particle that has one positive charge and is located in the nucleus is a neutron.
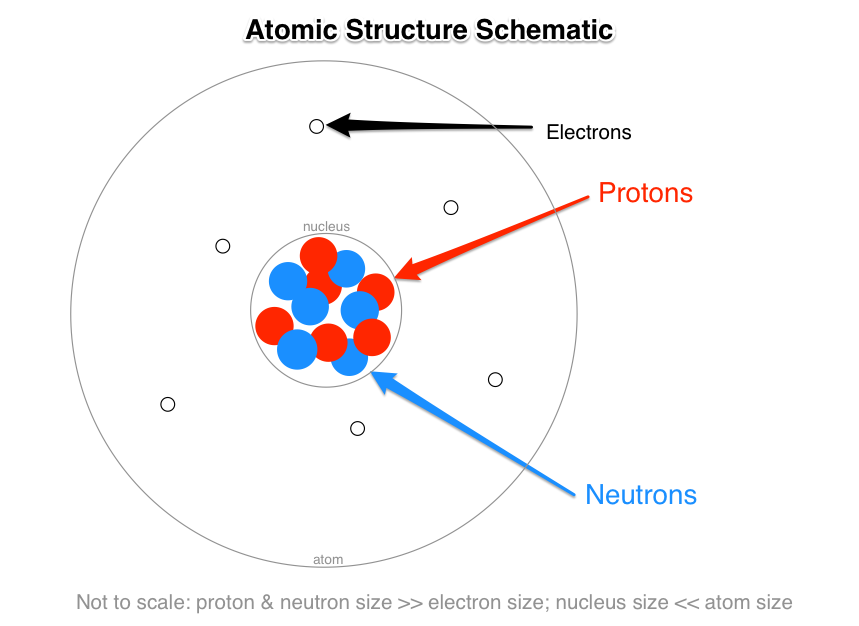 Source: socratic.org
Source: socratic.org
Protons and neutrons have the most mass. The nuclei of most atoms also contain neutrons. The nucleus contains two types of subatomic particles, protons and neutrons.
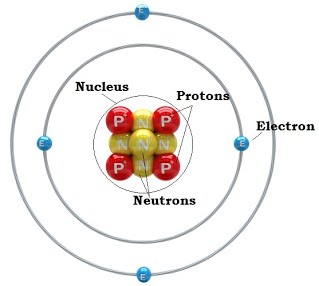 Source: 3galchemy.blogspot.com
Source: 3galchemy.blogspot.com
The protons and electrons b. Subatomic particles, the nucleus and isotopes. The nuclei of most atoms also contain neutrons.
 Source: sites.google.com
Source: sites.google.com
The center of the atom is called the nucleus. A subatomic particle is a unit of matter or energy that�s the fundamental makeup of all matter. The study of subatomic particles, atoms and molecules, and their structure and interactions, requires quantum mechanics.
Also Read :