Undissolved solute, if present, is in equilibrium with dissolved solvent d. An unsaturated solution is a very unstable solution b.
Which Statement Is True For A Saturated Solution. D) none of the statements is true. The number of moles of solute present in exactly one liter of solution is referred to as the. C) undissolved solute, if present, is continually dissolving. “a saturated solution can be quite dilute because some substances have very low solubility”.
 Solved Which Of The Following Statements Is True Of A | Chegg.com From chegg.com
Solved Which Of The Following Statements Is True Of A | Chegg.com From chegg.com
Related Post Solved Which Of The Following Statements Is True Of A | Chegg.com :
Solution saturation is reached when the rate at which a substance is dissolving is equal to the rate at which it is recrystallizing. Science chemistry q&a library which of the following statements concerning a saturated solution is incorrect? The solubility product values of agbr and agcl are 5 × 1 0 − 1 3 and 5 × 1 0 − 1 0 respectively. A solution where the solven cannot dissolve any more solute.
No more solute will dissolve in the solution.
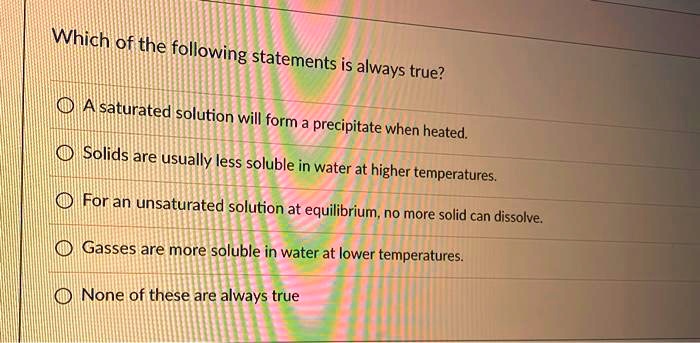
What is true of the ph of a solution that is an arrhenius acid? Solution saturation is reached when the rate at which a substance is dissolving is equal to the rate at which it is recrystallizing. The solution forms a precipitate. If the temperature is lowered, then the solubility of sugar will be reduced even further and the solution will continue to remain saturated. An unsaturated solution is a very unstable solution b. An unsaturated solution is a very unstable solution b.
 Source: chegg.com
Source: chegg.com
A carbonated beverge with bubbles. A saturated solution is a solution which is able to dissolve more solid ; For a salt in a solution, the concentration of ions will be in equilibrium.
 Source: chegg.com
Source: chegg.com
The solubility product values of agbr and agcl are 5 × 1 0 − 1 3 and 5 × 1 0 − 1 0 respectively. The solution has dissolved more solute than is generally possible, resulting in an unstable solution. A solubility of ‘20g per 100g of water’ means 100cm 3 of solution contains 20g of solid ;
 Source: clutchprep.com
Source: clutchprep.com
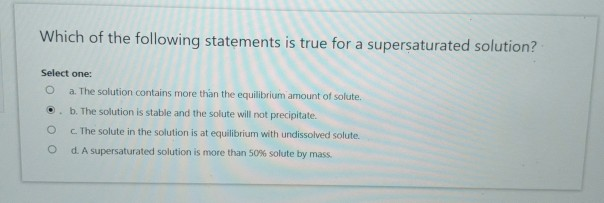
A supersaturated solution is more dilute than an unsaturated solution d. Which statements are true of any saturated solution at a given temperature? The concentration of pbcl2 is greater than normal.

Adding more solute will increase the saturation. A saturated solution need not to be a concentrated solution c. So in the given options [a g+]=[b r]+[c l] since a g is common.
 Source: chegg.com
Source: chegg.com
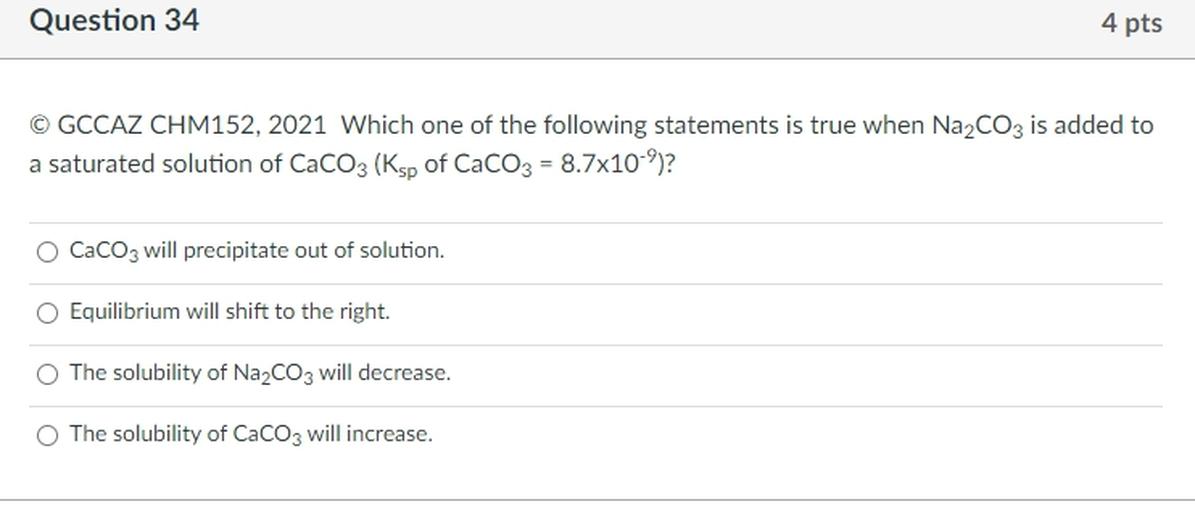
Consider a beaker containing a saturated solution of caf 2 in equilibrium with undissolved caf 2 (s).solid cacl 2 is then added to the solution. C) only one of the statements is true. A solubility of ‘20g per 100g of water’ means 100cm 3 of solution contains 20g of solid ;
An unsaturated solution is a very unstable solution b. A solution can be made less concentrated by adding additional solvent. A) the water table in the recharge area is at a higher elevation than the top of the aquifer in the subsurface.
 Source: questions-in.kunduz.com
Source: questions-in.kunduz.com
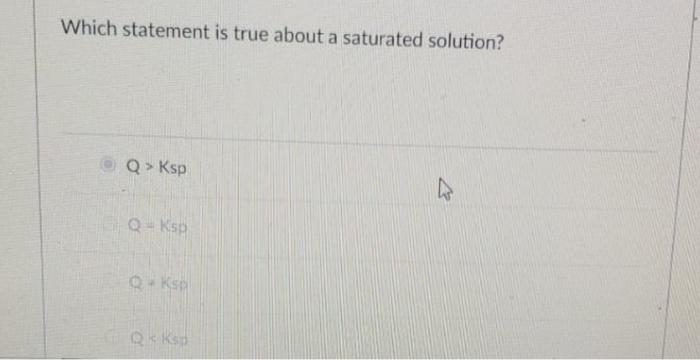
Which statements are true of any saturated solution at a given temperature? A saturated solution contains more than 100 g of dissolved solute. D) none of the statements is true.
 Source: bartleby.com
Source: bartleby.com
An unsaturated solution is a very unstable solution b. If a solution is saturated, it means that more solid will not dissolve in the solution at that temperature. Science chemistry q&a library which of the following statements concerning a saturated solution is incorrect?
 Source: numerade.com
Source: numerade.com
Solution saturation is reached when the rate at which a substance is dissolving is equal to the rate at which it is recrystallizing. B) undissolved solute may or may not be present. B) upward flow from a permeable aquitard is prevented by a confining aquifer.
 Source: slideplayer.com
Source: slideplayer.com
B) upward flow from a permeable aquitard is prevented by a confining aquifer. The solution forms a precipitate. So in the given options [a g+]=[b r]+[c l] since a g is common.
 Source: brainly.ph
Source: brainly.ph
C) only one of the statements is true. Undissolved solute may or may not be present c. Solution saturation is reached when the rate at which a substance is dissolving is equal to the rate at which it is recrystallizing.
 Source: brainly.ph
Source: brainly.ph
If the temperature is lowered, then the solubility of sugar will be reduced even further and the solution will continue to remain saturated. Undissolved solute may or may not be present c. Which of the following statements are true for a solution saturated with agcl & agbr.
 Source: brainly.ph
Source: brainly.ph
This is a true statement. A saturated solution is more concentrated than a supersaturated solution Which of the following statements describes a saturated solution?
 Source: yumpu.com
Source: yumpu.com
Adding more solute will increase the saturation. Consider a beaker containing a saturated solution of caf 2 in equilibrium with undissolved caf 2 (s).solid cacl 2 is then added to the solution. Which statements are true concerning a saturated solution of pbcl2?
 Source: numerade.com
Source: numerade.com
Undissolved solute may or may not be present c. If their solubilities in mole/litre present in separate solutions are x & y respectively. A supersaturated solution is more dilute than an unsaturated solution d.
 Source: numerade.com
Source: numerade.com
Undissolved solute, if present, is in equilibrium with dissolved solvent d. Consider a beaker containing a saturated solution of caf 2 in equilibrium with undissolved caf 2 (s).solid cacl 2 is then added to the solution. “a saturated solution can be quite dilute because some substances have very low solubility”.
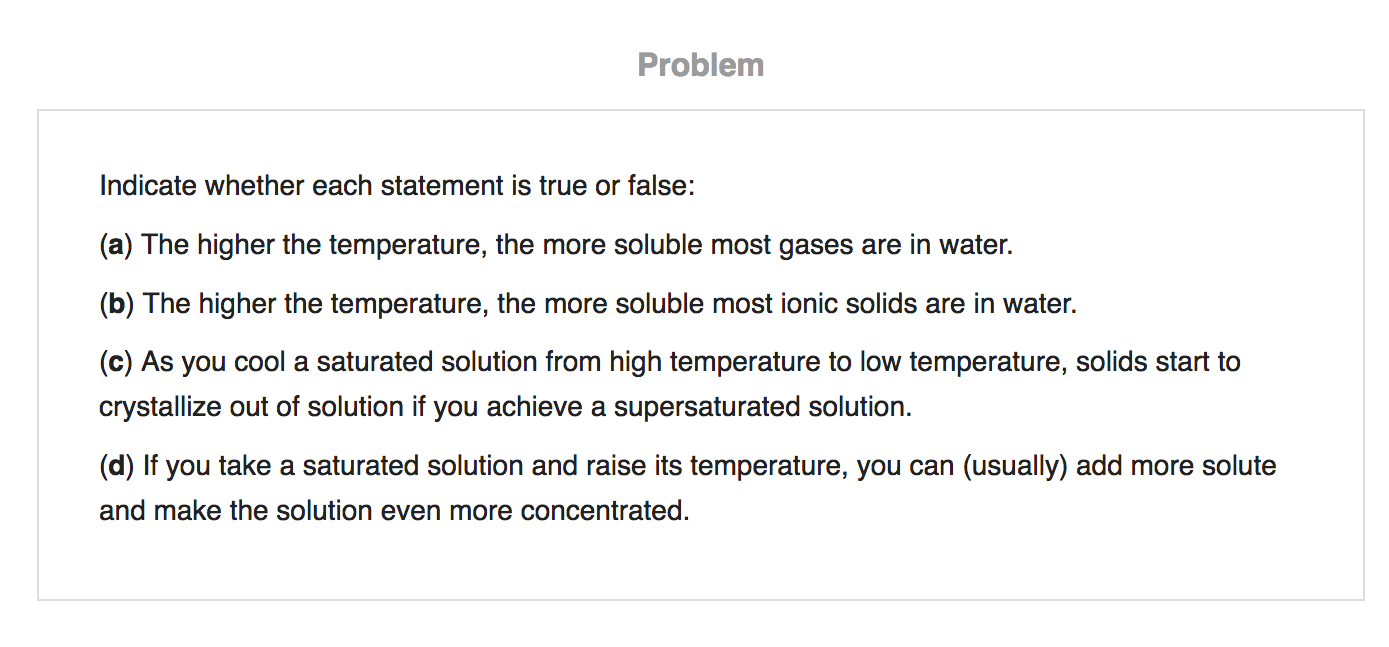 Source: chegg.com
Source: chegg.com
“a saturated solution can be quite dilute because some substances have very low solubility”. A solution of salt water with salt at the bottom. What is true of the ph of a solution that is an arrhenius acid?
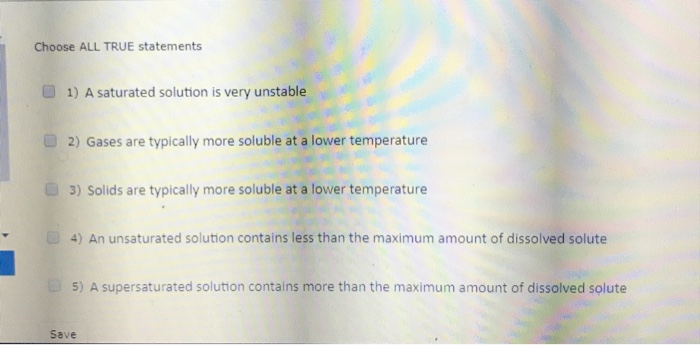
A supersaturated solution contains more solute at a given temperature than is needed to form a saturated solution. Consider a beaker containing a saturated solution of caf 2 in equilibrium with undissolved caf 2 (s).solid cacl 2 is then added to the solution. A solution of salt water with salt at the bottom.
 Source: slideplayer.com
Source: slideplayer.com
A solution where the solven cannot dissolve any more solute. The solution forms a precipitate. For example, the solubility of glucose at 25 °c is 91 g/100 ml of water.
 Source: brainly.ph
Source: brainly.ph
(1) a saturated solution always contains excess undissolved solute. This is a true statement. A solution of salt water with salt at the bottom.
Also Read :





