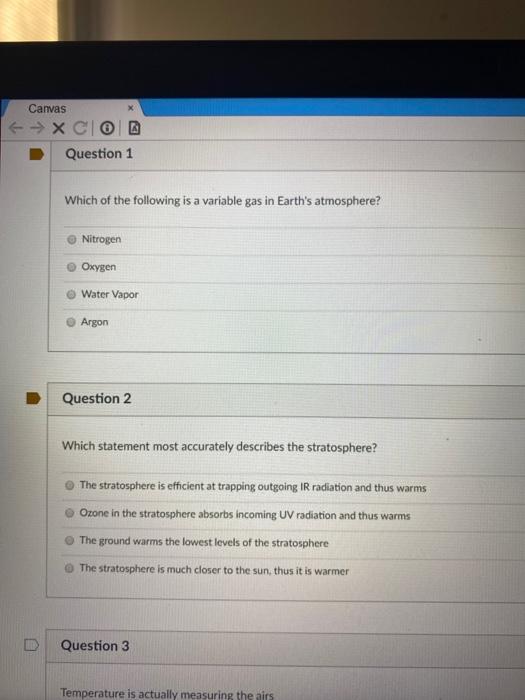
Which statement about the effusion rates of nitrogen and oxygen is true? Which of the following statements about monosaccharide structure is true?
Which Statement Is True About Nitrogen And Oxygen. Oxygen effuses 0.9 times faster than nitrogen. O they are both used to make water molecules. Which of the following statements correctly describes the typical number of bonds for carbon, nitrogen, and oxygen in most neutral organic molecules? Therefore, we can say that nitrogen has one lone pair of electrons.water contains 2 oxygen atoms bonded to hydrogen atoms.
 Solved Question 20 Which Of The Following Statements About | Chegg.com From chegg.com
Solved Question 20 Which Of The Following Statements About | Chegg.com From chegg.com
Related Post Solved Question 20 Which Of The Following Statements About | Chegg.com :
If a covalent bond were to be formed between a nitrogen atom (electronegativity 3.0) and an oxygen atom (electronegativity 3.5), which of the following statements would best describe such a bond? Animals breathe in nitrogen and release oxygen which is used by plants. They make up about 99.9% of the human body. Question 14 (1 point) formation of no from nitrogen and oxygen is an endothermic process.
It is a stable molecule despite having an unpaired electron.
They both contain no subatomic particles o they are both made of protons neutrons and electrons. They make up about 99.9% of the human body. Solve any question of classification of elements and periodicity in. So what is air, exactly? O they are both used to make water molecules. What statements is true regarding.
 Source: doubtnut.com
Source: doubtnut.com
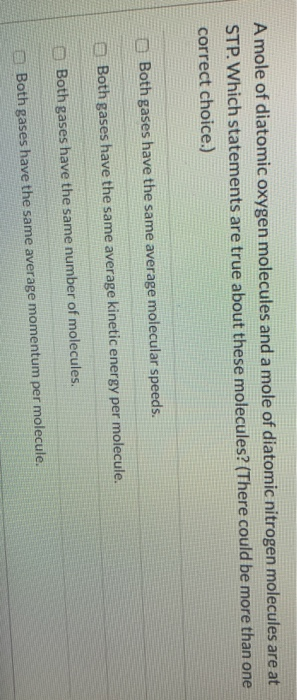
The first statement is valence electrons are shared between nitrogen and oxygen atoms. Oxygen effuses 1.07 times faster than nitrogen. Which of the following statements is true about the bonds that are broken and formed during this reaction?
An increase in the content of harmful substance (pollutants) in the air like carbon dioxide, carbon monoxide, oxides of sulphur, nitrogen, fluoride, lead, nickel, arsenic and dust particles etc. The nitrogen has two lone pairs of electrons. Which of the following statements correctly describes the typical number of bonds for carbon, nitrogen, and oxygen in most neutral organic molecules?
 Source: doubtnut.com
Source: doubtnut.com
Oxygen effuses 1.07 times faster than nitrogen. Animals breathe in oxygen and release carbon dioxide which is used by plants. Aldoses and ketoses differ in the position of their hydroxyl groups.
 Source: chegg.com
Source: chegg.com
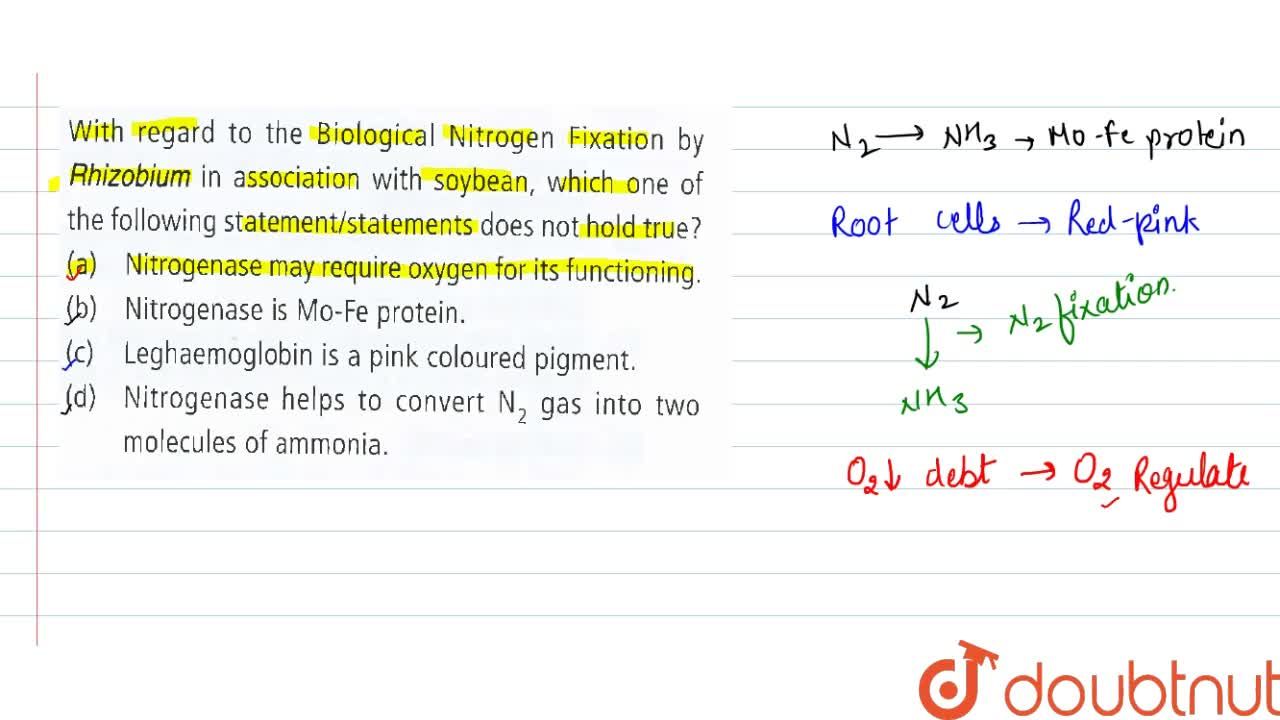
Which of the given statements are true regarding a co valent bond between nitrogen and oxygen atoms in a nitric oxide molecule, nitric oxide. Oxygen effuses 0.9 times faster than nitrogen. Question 8 (4 points) formation of no from nitrogen and oxygen is an endothermic process.

Animals breathe in oxygen and release carbon dioxide which is used by plants. Question 8 (4 points) formation of no from nitrogen and oxygen is an endothermic process. Neutron quantities vary, since there is more than one isotope of both oxygen and nitrogen.
 Source: doubtnut.com
Source: doubtnut.com
They are both made of portions, neutrons, and electrons. When an oxygen atom accepts bond pairs of electrons due to heterolytic bond cleavage, it has a formal charge of. Neutron quantities vary, since there is more than one isotope of both oxygen and nitrogen.

Oxygen contains 6 electrons in its valence orbitals which means that out of the 6 electrons, two electrons will. They both contain no subatomic particles o they are both made of protons neutrons and electrons. A.) carbon, oxygen, sodium, and hydrogen b.) carbon, hydrogen, nitrogen, and oxygen c.) carbon, phosphorus, sodium, and hydrogen d.) carbon, calcium, oxygen, and nitrogen e.) carbon, sodium, chlorine, and oxygen
 Source: chegg.com
Source: chegg.com
Which of the following statements correctly describes the typical number of bonds for carbon, nitrogen, and oxygen in most neutral organic molecules? Which of the following statements is true about the bonds that are broken and formed during this reaction? Which of the following statements is true about the bonds that are broken and formed during this reaction?
 Source: chegg.com
Source: chegg.com
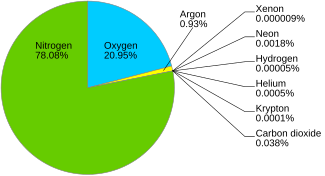
The wait in earth’s environment is comprised of around 78 percent nitrogen and 21 percent oxygen. An increase in the content of harmful substance (pollutants) in the air like carbon dioxide, carbon monoxide, oxides of sulphur, nitrogen, fluoride, lead, nickel, arsenic and dust particles etc. Therefore, we can say that nitrogen has one lone pair of electrons.water contains 2 oxygen atoms bonded to hydrogen atoms.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The second ionisation energy of oxygen is greater than that of nitrogen. Carbon forms 4 covalent bonds, nitrogen forms 3 covalent bonds and oxygen forms 2 covalent bonds Which of the given statements are true regarding a co valent bond between nitrogen and oxygen atoms in a nitric oxide molecule, nitric oxide.
 Source: byjus.com
Source: byjus.com
Which of the following statements about monosaccharide structure is true? The first statement is valence electrons are shared between nitrogen and oxygen atoms. Which of the following statements is true about the bonds that are broken and formed during this reaction?
 Source: chegg.com
Source: chegg.com
The following that are true regarding ionosphere are the molecules and atoms of nitrogen and oxygen in this layer absorb a part of solar radiation and become ionized and this layer is responsible for reflecting back the radio wave transmitted from the earth. Nitrogen effuses 1.07 times faster than oxygen. When a nitrogen atom forms three covalent bonds and one coordinate bond, it has formal charge of +1.
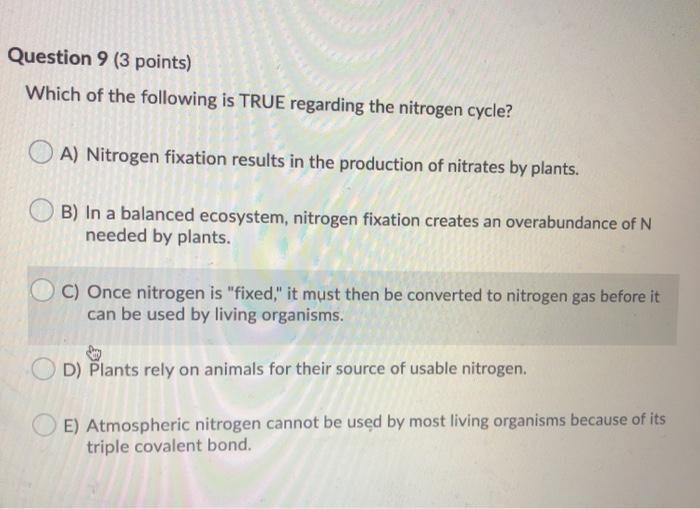
Which of the following statements correctly describes the typical number of bonds for carbon, nitrogen, and oxygen in most neutral organic molecules? Animals breathe in nitrogen and release carbon dioxide which is used by plants. In this question, we have pride.
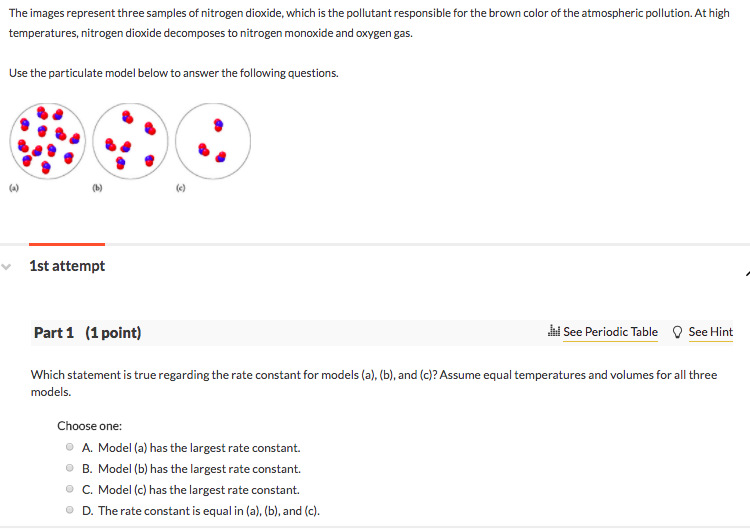 Source: chegg.com
Source: chegg.com
They are both made of portions, neutrons, and electrons. Question 14 (1 point) formation of no from nitrogen and oxygen is an endothermic process. They make up about 99.9% of the human body.

Which of the given statements are true regarding a co valent bond between nitrogen and oxygen atoms in a nitric oxide molecule, nitric oxide. The first statement is valence electrons are shared between nitrogen and oxygen atoms. Question 14 (1 point) formation of no from nitrogen and oxygen is an endothermic process.
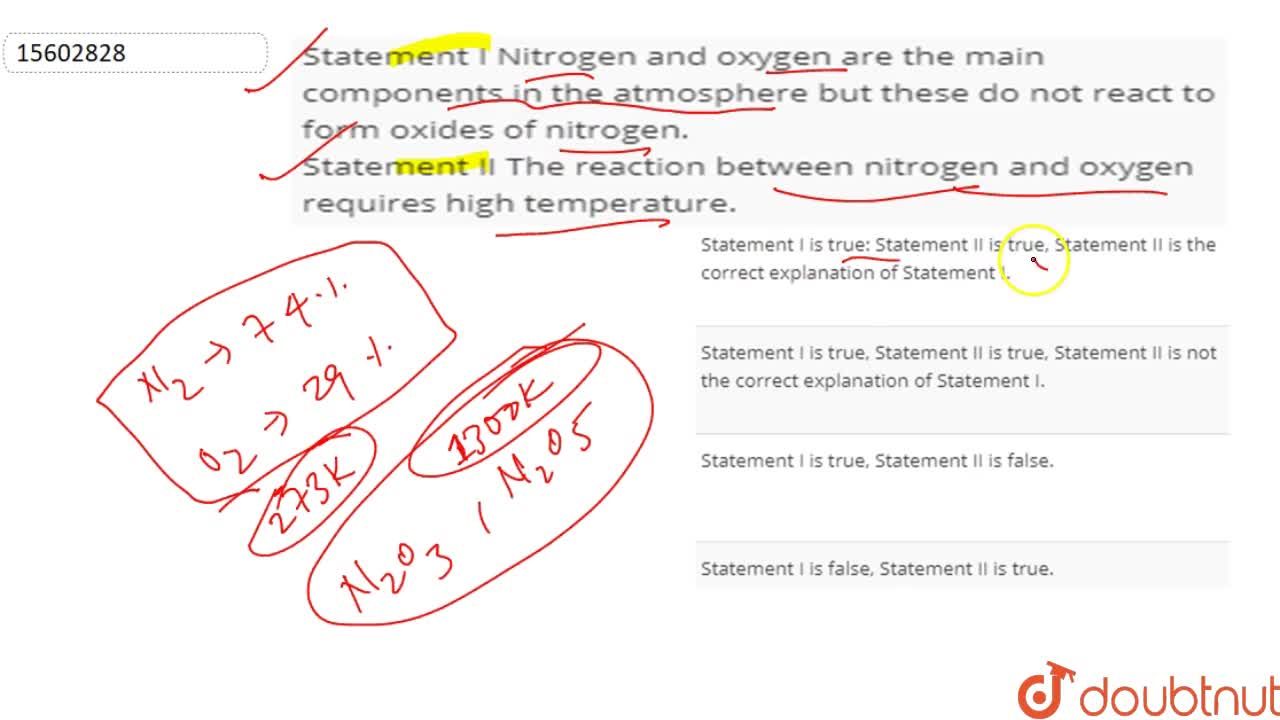 Source: doubtnut.com
Source: doubtnut.com
N2(g) + o2(g) → 2no(g) the products have a lower potential energy because the bonds in the products are stronger than those in the reactants the products have a Oxygen has 8 electrons and can form two bonds with other atoms. Question 8 (4 points) formation of no from nitrogen and oxygen is an endothermic process.

The following that are true regarding ionosphere are the molecules and atoms of nitrogen and oxygen in this layer absorb a part of solar radiation and become ionized and this layer is responsible for reflecting back the radio wave transmitted from the earth. Air additionally has tiny amounts of many other gases, too, such as carbon dioxide, neon, and also hydrogen. Oxygen has 8 electrons and can form two bonds with other atoms.
 Source: slideplayer.com
Source: slideplayer.com
Monosaccharides can be classified according to the spatial arrangement of their atoms. Carbon forms 4 covalent bonds, nitrogen forms 2 covalent bonds and oxygen forms 3 covalent bonds b. Question 22 (4 points) formation of no from nitrogen and oxygen is an endothermic process.
 Source: youtube.com
Source: youtube.com
Which statement about the effusion rates of nitrogen and oxygen is true? The wait in earth’s environment is comprised of around 78 percent nitrogen and 21 percent oxygen. The second ionisation energy of oxygen is greater than that of nitrogen.
 Source: chegg.com
Source: chegg.com
N2(g) + o2(g) → 2no(g) the products have a lower potential energy because the bonds in the products are stronger than those in the reactants the products have a Which of the following statements about monosaccharide structure is true? They both contain no subatomic particles o they are both made of protons neutrons and electrons.
Also Read :





