(b) are attracted to each other by strong forces. According to kinetic molecular theory,which of the following statements is true of the molecules in the gaseous state?
Which Statement Is True About Kinetic Molecular Theory. Which statement about the molecules in the gas carbon dioxide is correct? Their size is assumed to be much smaller than the average distance between the particles. Molecules of different gases with the same mass and. (b) are attracted to each other by strong forces.

Related Post Solved Which Statement Is True About Kinetic Molecular | Chegg.com :
Completely elastic_ energy of a particle is not proportional to the temperature: According to the kinetic molecular theory which statement describes the particles of an ideal gas; A) the collisions of particles with one another is completely elastic b) the size of the particle is large compared to the volume c) a single particle does not move in a straight line d) the average kinetic energy of a particle is not proportional to the temperature Which statement is true about kinetic molecular theory?
Kinetic molecular theory can be used to explain both charles’ and boyle’s laws.
Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. Particle docs not move in straight line a single of the particle is large compared t0 the volume the size the collisions of particles with one another : Gas particles are constantly colliding with each other and the walls of their container. Their size is assumed to be much smaller than the average distance between the particles. (4 points) matter consists of only large molecules. According to kinetic molecular theory,which of the following statements is true of the molecules in the gaseous state?
![Solved:which Of The Statements Below Is True Of Kinetic Molecular Theory? [Select All That Apply] Gas Particles Have A Negligible Volume Gases Are Compressible Collisions Of Gas Particles Are Elastic (Perfect Collisions) Solved:which Of The Statements Below Is True Of Kinetic Molecular Theory? [Select All That Apply] Gas Particles Have A Negligible Volume Gases Are Compressible Collisions Of Gas Particles Are Elastic (Perfect Collisions)](https://cdn.numerade.com/ask_images/4e521faee0da40c1aa0f99ad9bb3ff2a.jpg) Source: numerade.com
Source: numerade.com
The average kinetic energy of a particle is not proportional to the temperature. This is one of the postulates of the kinetic theory of matter. The temperature of a substance is a measure of the average kinetic energy of the particles.
 Source: chegg.com
Source: chegg.com
Based on the kinetic theory, which statement is true? A) the collisions of particles with one another is completely elastic b) the size of the particle is large compared to the volume c) a single particle does not move in a straight line d) the average kinetic energy of a particle is not proportional to the temperature The size of the particle is large compared to the volume.
 Source: chegg.com
Source: chegg.com
The particles of matter are in constant motion. Which statement is true according to the kinetic theory? The temperature of a substance is a measure of the average kinetic energy of the particles.
 Source: chegg.com
Source: chegg.com
Kinetic explanation of avogadro’s law: According to kinetic molecular theory,which of the following statements is true of the molecules in the gaseous state? Which of the following statements about the kinetic particle theory is not true?
 Source: chegg.com
Source: chegg.com
According to kinetic molecular theory,which of the following statements is true of the molecules in the gaseous state? The theory assumes that gases consist of widely separated molecules of negligible volume that are in constant motion, colliding elastically with one another and the walls of their container with average velocities determined by their absolute. (a) according to the kinetic molecular theory, gas particles have mass but no volume.
 Source: clutchprep.com
Source: clutchprep.com
(b) are attracted to each other by strong forces. If we increase the number of gas molecules in a closed container, more of them will collide with the walls per unit time. According to the kinetic molecular theory collisions between gas particles are perfectly elastic.
 Source: doubtnut.com
Source: doubtnut.com
The size of the particle is large compared to the volume. Based on the kinetic theory, which statement is true? Matter is made up of particles that are constantly moving.
 Source: numerade.com
Source: numerade.com
Matter consists of only large molecules. Which statement is true according to the kinetic molecular theory? That is, there is no net loss of energy from the collisions.
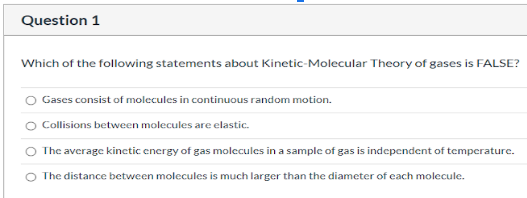 Source: clutchprep.com
Source: clutchprep.com
According to the kinetic molecular theory collisions between gas particles are perfectly elastic. (c) according to the kinetic molecular theory, when gas particles collide, they bounce off. Kinetic energy is the energy of mass in motion.
 Source: chegg.com
Source: chegg.com
The molecules are close together. The density of a gas is usually comparable to the density of solids. Particle docs not move in straight line a single of the particle is large compared t0 the volume the size the collisions of particles with one another :
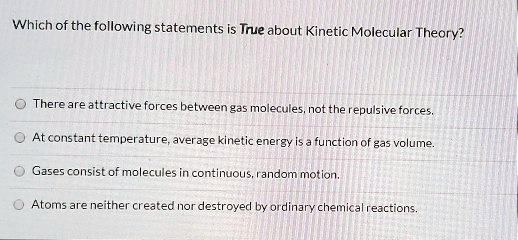 Source: numerade.com
Source: numerade.com
The kinetic molecular theory describes a gas as a collection of the large number of identical submicroscopic particles (atoms or molecules), all of which are in constant, rapid, random motion. The particles undergo random elastic collisions between themselves and with the. The molecules are close together.
 Source: clutchprep.com
Source: clutchprep.com
The theory assumes that gases consist of widely separated molecules of negligible volume that are in constant motion, colliding elastically with one another and the walls of their container with average velocities determined by their absolute. All particles have energy, but the energy varies depending on the temperature the sample of matter is in. Which statement is true about kinetic molecular theory?
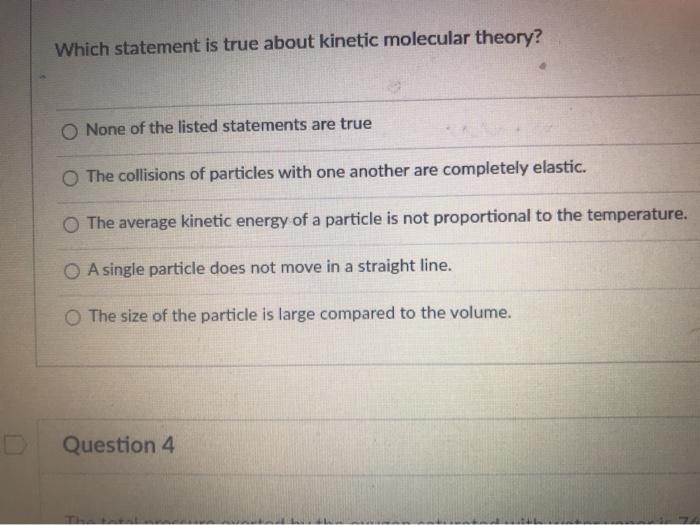
The particles of matter are in constant motion. The average kinetic energy of a particle is not proportional to the temperature. C) the collisions of particles with one another is not completely elastic.

The average kinetic energy of a collection of gas particles is directly proportional to absolute temperature only. All particles have energy, but the energy varies depending on the temperature the sample of matter is in. The kinetic molecular theory describes a gas as a collection of the large number of identical submicroscopic particles (atoms or molecules), all of which are in constant, rapid, random motion.
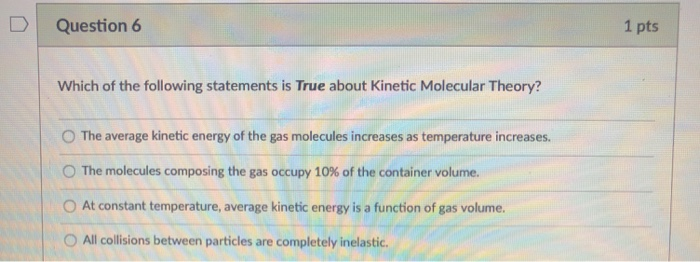 Source: chegg.com
Source: chegg.com
Kinetic energy is the energy of mass in motion. Molecules of different gases with the same mass and. The kinetic molecular theory of gases is a model that helps us understand the physical properties of gases at the molecular level.
 Source: chegg.com
Source: chegg.com
Quesliun which statement is true about kinetic molecular theory? The kinetic molecular theory is based on the following postulates, or assumptions. A) a single particle does not move in a straight line.
 Source: clutchprep.com
Source: clutchprep.com
In the kinetic molecular theory of gas behavior, the assumption is made that gas molecules (a) move rapidly in random directions. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. The particles of matter are in constant motion.
 Source: slideplayer.com
Source: slideplayer.com
The particles of matter are in constant motion. (c) according to the kinetic molecular theory, when gas particles collide, they bounce off. Answer expert verified the true statement is that different gases with the same mass and temperature always have the same average kinetic energy.
 Source: studylib.net
Source: studylib.net
D) the average kinetic energy of a particle is proportional to the temperature in kelvin. (c) according to the kinetic molecular theory, when gas particles collide, they bounce off. The particles of matter have zero kinetic energy.
 Source: numerade.com
Source: numerade.com
A single particle does not move in a straight line. The average kinetic energy of a particle is not proportional to the temperature. This is one of the postulates of the kinetic theory of matter.
Also Read :





