We can use quantum mechanics to predict the specific regions around an atom where electrons are likely to be located: According to the vsepr model, which molecule has a predicted tetrahedral molecular geometry?
Which Statement Is Always True According To Vsepr Theory. If you are having trouble with chemistry, organic, physics, calculus, or statistics, we got your back! Which statement is always true according to vsepr theory? The theory says that electrons fill the atomic orbitals of an atom within a molecule. Quiz 11 :chemical bonding ii:
 Solved:which Statement Is Always True According To Vsepr Theory? The Preferred Geometry Of_ Molecule Is The One In Which The Lectron Groups Have The Maximum Energy Possible_ The Preferred Geometry Of_ Molecule From numerade.com
Solved:which Statement Is Always True According To Vsepr Theory? The Preferred Geometry Of_ Molecule Is The One In Which The Lectron Groups Have The Maximum Energy Possible_ The Preferred Geometry Of_ Molecule From numerade.com
Related Post Solved:which Statement Is Always True According To Vsepr Theory? The Preferred Geometry Of_ Molecule Is The One In Which The Lectron Groups Have The Maximum Energy Possible_ The Preferred Geometry Of_ Molecule :
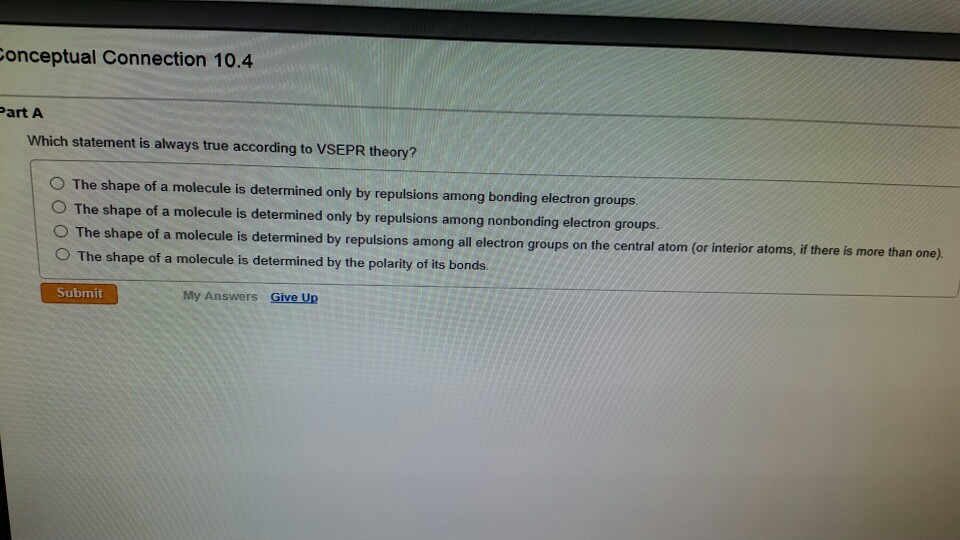
The shape of a molecule is determined by repulsions among all electron groups on the central atom (or interior atoms, if there is more than one). Molecular orbitals are formed by adding and. Quiz 11 :chemical bonding ii: Which statement is always true according to vsepr theory?
The theory says that electrons fill the atomic orbitals of an atom within a molecule.
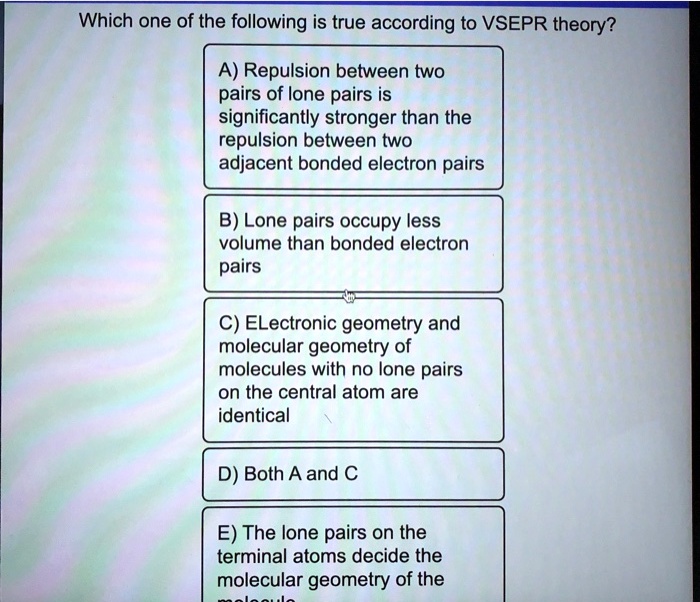
A) the shape of a molecule is determined by the polarity of its bonds. Repulsions between only bonding electron groups on the central atom determine the geometry of a molecule. Quiz 11 :chemical bonding ii: Which statement is always true according to vsepr theory? The polarity of the bonds determine the geometry of a molecule. Valence shell electron pair repulsion theory (vsepr theory) 14.
 Source: researchgate.net
Source: researchgate.net
The shape of a molecule is determines by repulsions among all electron groups on the central atom (or interior atoms, if there is more than one). Predicting the shapes of molecules. Our videos prepare you to succeed in your college classes.
 Source: numerade.com
Source: numerade.com
Which one of the following statements is always true according to vsepr theory? Yes, and you draw the dipole moment pointing between the green atoms, the fluorines. July 13, 2021 july 13, 2021 thanh.
 Source: numerade.com
Source: numerade.com
What does “ they can drive it or milk it “ mean? The polarity of the bonds determine the geometry of a molecule. Let us help you simplify your studying.

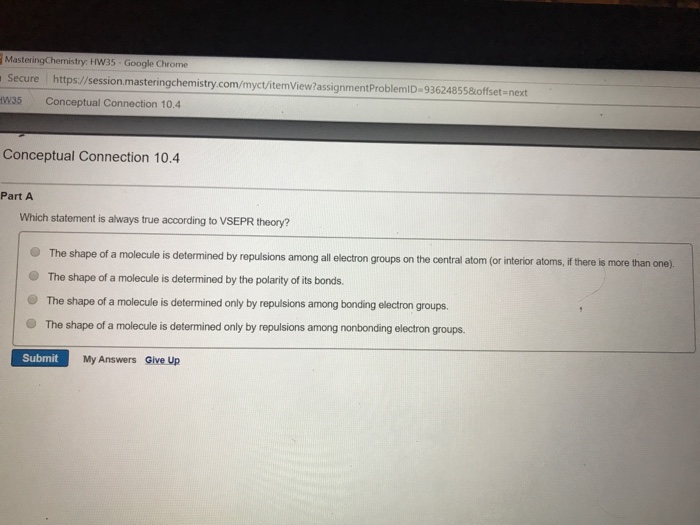
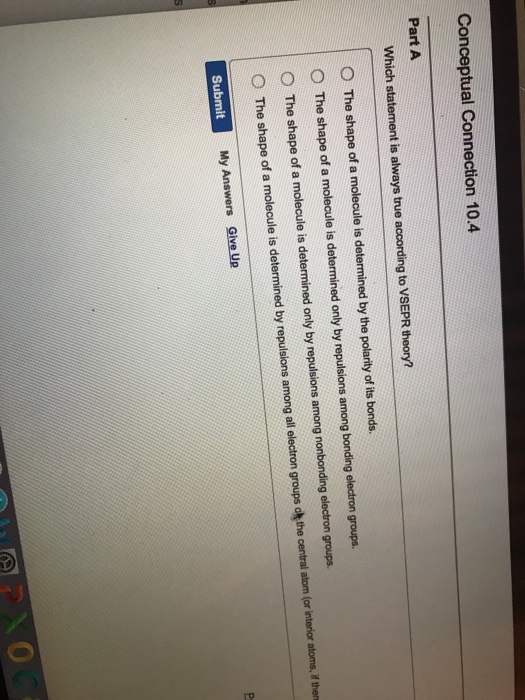
Which statement is always true according to vsepr theory?a) the shape of a molecule is determined only by the repulsions among bonding electron groups.b) the shape of a molecule is determined by the repulsions among all electron groups.c) the shape of a molecule is determined only by the repulsions among nonbonding electron groups.d) the shape of a. Yes, and you draw the dipole moment pointing between the green atoms, the fluorines. Now, we move on and look at the various postulates of the valence bond theory.
Which statement is always true according to vsepr theory? A) the shape of a molecule is determined by the polarity of its bonds. No, there are 4 atoms attached.
![Solved:quiz Chap9 Chemi0I-201 Id: Name: Scc: S.n. According To The Vsepr Model: The Molecular Geometry Of Brfs Is Square Planar [True Or False]: According To The Vsepr Model;, The Clectron Domain Geometry Solved:quiz Chap9 Chemi0I-201 Id: Name: Scc: S.n. According To The Vsepr Model: The Molecular Geometry Of Brfs Is Square Planar [True Or False]: According To The Vsepr Model;, The Clectron Domain Geometry](https://cdn.numerade.com/ask_images/92ac837ae985452eba954e6b1b2800ed.jpg) Source: numerade.com
Source: numerade.com
July 13, 2021 july 13, 2021 thanh. Now, we move on and look at the various postulates of the valence bond theory. July 13, 2021 july 13, 2021 thanh.
 Source: chegg.com
Source: chegg.com
According to the vsepr theory, the molecular geometry of the molecule is linear. The valence shell electron pair repulsion theory (vsepr) states that “whenever there is a repulsion between the pairs of valence electrons in all atoms, the atoms will arrange themselves in a geometric shape so as to minimize the electron pair repulsion.” the aim is that the molecule must have minimum energy and maximum stability. Which statement is always true according to vsepr theory?
There are successful theories that describe the electronic structure of atoms. Which statement is always true according to vsepr theory? The preferred geometry of a molecule is the one in which the electron groups have the maximum energy possible.

Xef2 is nonpolar due to the symmetric arrangement of the bonded pairs of electrons. Chemistry q&a library which statement is always true according to vsepr theory?(a) the shape of a molecule is determined only by repulsions among bonding electron groups.(b) the shape of a molecule is determined only by repulsions among nonbonding electron groups.(c) the shape of a molecule is determined by the polarity of its bonds.(d) the shape of a molecule is determined. The shape of a molecule is determines by repulsions among all electron groups on the central atom (or interior atoms, if there is more than one).
 Source: en.wikipedia.org
Source: en.wikipedia.org
A double bond is two sigma bonds. Molecular orbitals are formed by adding and. The shape of a molecule is determines by repulsions among all electron groups on the central atom (or interior atoms, if there is more than one).

What does “ they can drive it or milk it “ mean? Repulsions between all electron groups on the central atom determine the geometry of a molecule. Which statement is always true according to vsepr theory?
 Source: numerade.com
Source: numerade.com
Which statement is always true according to vsepr theory?a) the shape of a molecule is determined only by the repulsions among bonding electron groups.b) the shape of a molecule is determined by the repulsions among all electron groups.c) the shape of a molecule is determined only by the repulsions among nonbonding electron groups.d) the shape of a. Which statement is always true according to vsepr theory? Which of the following is a correct statement about mrna;
 Source: howtodiscuss.com
Source: howtodiscuss.com
Predicting the shapes of molecules. Repulsions between only bonding electron groups on the central atom determine the geometry of a molecule. The shape of a molecule is determined only by repulsions among bonding electron groups.
Our videos will help you understand concepts, solve your homework, and do great on your exams. As there are fluorine molecules on both the side of the central atom, there is no dipole moment and hence there is no polarity. Identify the choice that best completes the statement or answers the question.
 Source: quizlet.com
Source: quizlet.com
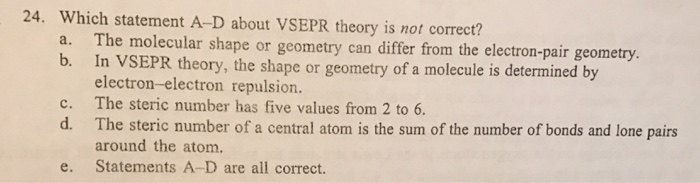
Which statement is always true according to vsepr theory? (1) both tetrahedral and trigonal pyramidal molecules have four vsepr electron groups. The valence shell electron pair repulsion theory (vsepr) states that “whenever there is a repulsion between the pairs of valence electrons in all atoms, the atoms will arrange themselves in a geometric shape so as to minimize the electron pair repulsion.” the aim is that the molecule must have minimum energy and maximum stability.

Which one of the following statements is always true according to vsepr theory? Valence bond theory describes the electronic structure of molecules. Which statement is always true according to vsepr theory?(a) the shape of a molecule is determined only by repulsions among bonding electron groups.(b) the shape of a molecule is determined only by repulsions among nonbonding electron groups.(c) the shape of a molecule is determined by the polarity of its bonds.(d) the shape of a molecule is determined by.
 Source: chegg.com
Source: chegg.com
We can use quantum mechanics to predict the specific regions around an atom where electrons are likely to be located: Now, we move on and look at the various postulates of the valence bond theory. Which statement is always true according to vsepr theory?a) the shape of a molecule is determined only by the repulsions among bonding electron groups.b) the shape of a molecule is determined by the repulsions among all electron groups.c) the shape of a molecule is determined only by the repulsions among nonbonding electron groups.d) the shape of a.

Our videos will help you understand concepts, solve your homework, and do great on your exams. The shape of a molecule is determined only by repulsions among bonding electron groups. Let us help you simplify your studying.
Predicting the shapes of molecules. For a molecule with the formula ab 2 the molecular shape is _____. Yes, and you draw the dipole moment pointing between the white balls, the hydrogens.
 Source: chegg.com
Source: chegg.com
According to the vsepr theory, the geometry of the so 3 molecule is a) pyramidal. The geometry of the cs 2 molecule is best described as a) linear. According to the vsepr model, which molecule has a predicted tetrahedral molecular geometry?
Also Read :