It is in the same phase as the reactants. It forms different products than the uncatalyzed reaction forms.
Which Statement Describes A Homogeneous Catalyst. Which statement describes a homogeneous catalyst? It is in the same phase as the reactants. Chemical catalysts can react with a variety of substrates. It is completely consumed by reactants.
 Environmentally Benign Production Of Biodiesel Using Heterogeneous Catalysts - Hara - 2009 - Chemsuschem - Wiley Online Library From chemistry-europe.onlinelibrary.wiley.com
Environmentally Benign Production Of Biodiesel Using Heterogeneous Catalysts - Hara - 2009 - Chemsuschem - Wiley Online Library From chemistry-europe.onlinelibrary.wiley.com
Related Post Environmentally Benign Production Of Biodiesel Using Heterogeneous Catalysts - Hara - 2009 - Chemsuschem - Wiley Online Library :
It is completely consumed by reactants. It is in the same phase as the reactants. It is in the same phase as the reactants. A catalyst does not affect the thermodynamic parameters of a reaction such as enthalpy, entropy, gibbs energy, and equilibrium constant.
It is completely consumed by reactants.
It is in the same phase as the reactants. As catalyst increases the reaction rate by lowering the activation energy, so, option a is correct. It interacts with a reactant to form an intermediate substance, which then decomposes or reacts with another reactant in one or more steps to regenerate the original catalyst and form product. It provides an alternative pathway for the reaction. It is completely consumed by reactants. Which statement describes a homogeneous catalyst?
 Source: chemistry-europe.onlinelibrary.wiley.com
Source: chemistry-europe.onlinelibrary.wiley.com
The portion of an enzyme that binds substrate and carries out the actual catalysis is termed the active site. It is a homogeneous catalyst because both the reactant and the enzyme are liquids. Which statement describes a catalyst?
 Source: onlinelibrary.wiley.com
Source: onlinelibrary.wiley.com
Catalytic converters that contain solid platinum, palladium, and rhodium change these poisonous gases into harmless gases. It is in the same phase as the reactants. It is in the same phase as the reactants.
 Source: brainly.in
Source: brainly.in
They are (1) homogeneous, (2) heterogeneous (solid), (3) heterogenized homogeneous catalyst and (4. B.the products will be contaminated. It is in a gaseous phase only.
 Source: slideserve.com
Source: slideserve.com
It is a homogeneous catalyst because both the reactant and the enzyme are liquids. Which statement describes a homogeneous catalyst? They are (1) homogeneous, (2) heterogeneous (solid), (3) heterogenized homogeneous catalyst and (4.
 Source: brainly.in
Source: brainly.in
It is in a gaseous phase only. A homogeneous catalyst is present in the same phase as the reactants. Which statement best describes how a catalyst can speed up a chemical reaction?
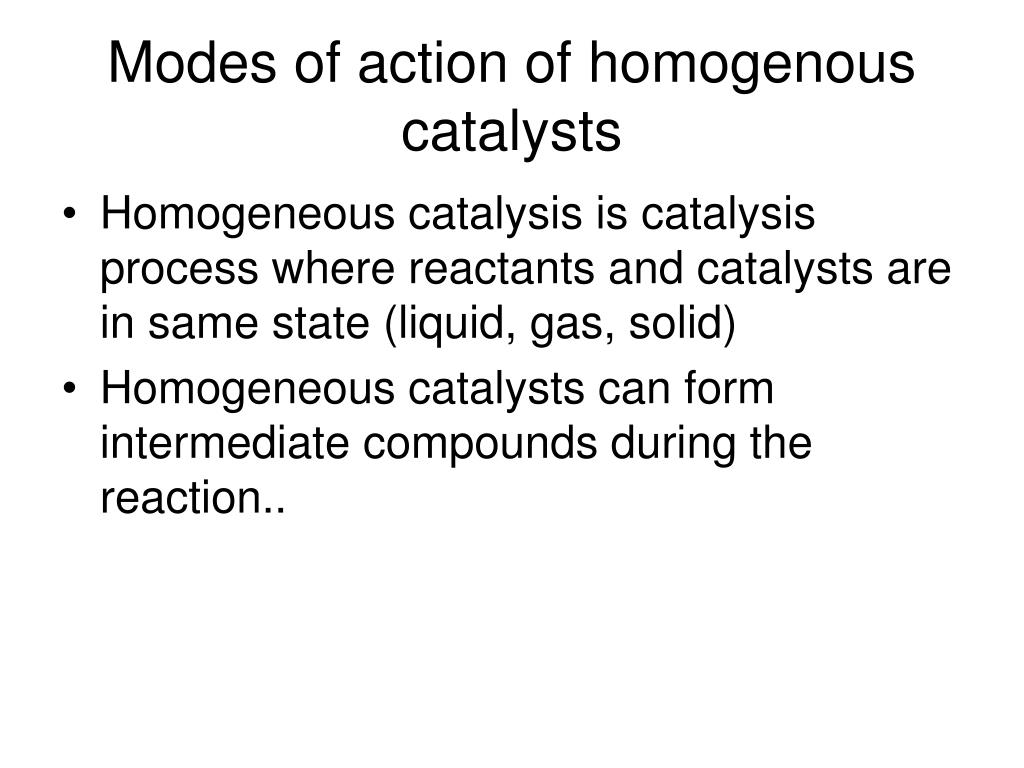
 Source: mdpi.com
Source: mdpi.com
Heteroimplies different (as in heterosexual). It forms different products than the uncatalyzed reaction forms. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution.
 Source: mdpi.com
Source: mdpi.com
It is the difference between reactant energy and maximum energy. Homogeneous catalysts are those that occupy the same phase as the reaction mixture (typically liquid or gas ) while heterogeneous catalysts occupy a different phase. What overall effect does adding a catalyst have on a chemical reaction?
 Source: slideserve.com
Source: slideserve.com
Which statement about the catalysts used is correct? As catalyst increases the reaction rate by lowering the activation energy, so, option a is correct. The amount of catalyst is the same at the end as at the beginning of the reaction.
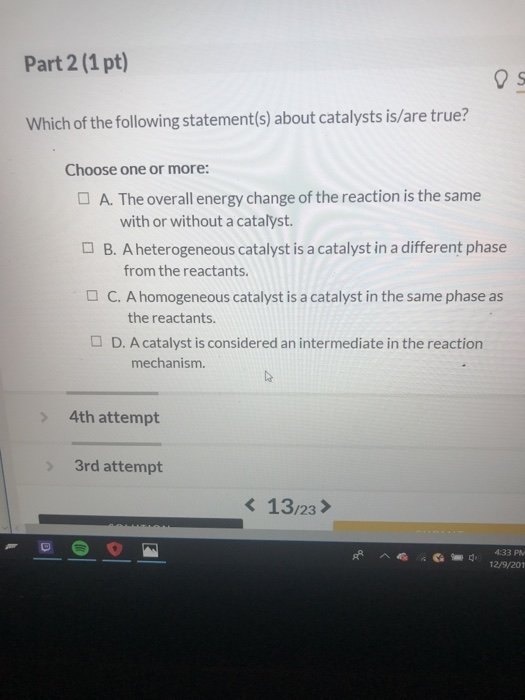 Source: chegg.com
Source: chegg.com
It lowers the activation energy that is needed. Catalysts provide a lower energy pathway for the reaction. It forms different products than the uncatalyzed reaction forms.
 Source: quizlet.com
Source: quizlet.com
It is in a gaseous phase only. Which statement describes a homogeneous catalyst? B.the products will be contaminated.
 Source: edubrainly.com
Source: edubrainly.com
Catalytic converters that contain solid platinum, palladium, and rhodium change these poisonous gases into harmless gases. D reaction 1 uses a homogeneous catalyst and reaction 2 uses a heterogeneous catalyst. It is in a gaseous phase only.
 Source: studylib.net
Source: studylib.net
A.the catalyst will become heterogeneous. What overall effect does adding a catalyst have on a chemical reaction? It forms different products than the uncatalyzed reaction forms.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A.the catalyst will become heterogeneous. Which of the following most likely will occur if a homogeneous catalyst cannot be separated from the products at the end of a reaction? It is the difference between reactant energy and maximum energy.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is in the same phase as the reactants. It is a homogeneous catalyst because both the reactant and the enzyme are liquids. The portion of an enzyme that binds substrate and carries out the actual catalysis is termed the active site.
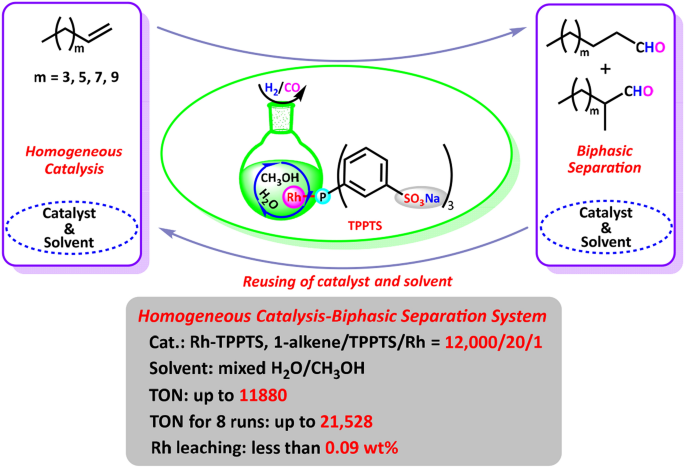 Source: link.springer.com
Source: link.springer.com
Which statement describes a homogeneous catalyst? Which statement describes a catalyst? It forms different products than the uncatalyzed reaction forms.
 Source: brainly.com
Source: brainly.com
Which statement describes a homogeneous catalyst? They are (1) homogeneous, (2) heterogeneous (solid), (3) heterogenized homogeneous catalyst and (4. The portion of an enzyme that binds substrate and carries out the actual catalysis is termed the active site.
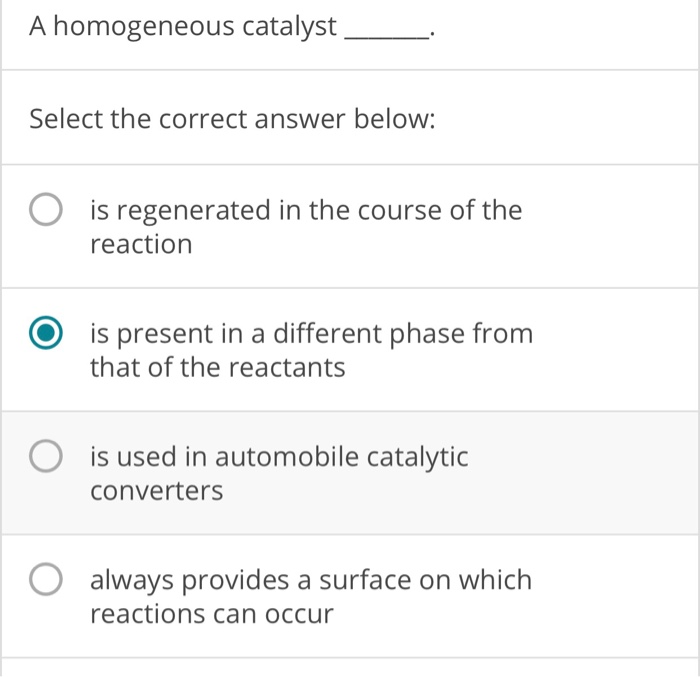 Source: chegg.com
Source: chegg.com
The amount of catalyst is the same at the end as at the beginning of the reaction. It is in a gaseous phase only. It is in a gaseous phase only.
 Source: clutchprep.com
Source: clutchprep.com
They are (1) homogeneous, (2) heterogeneous (solid), (3) heterogenized homogeneous catalyst and (4. It forms different products than the uncatalyzed reaction forms. Which statement defines activation energy?
 Source: quizlet.com
Source: quizlet.com
A homogeneous catalyst is present in the same phase as the reactants. Which statement describes a homogeneous catalyst? Which statement describes a homogeneous catalyst?

It is in the same phase as the reactants. A catalyst increases the rate of the reaction by lowering the activation energy. It is in the same phase as the reactants.
Also Read :