A triple bond is less stable or has a lower bond dissociation energy than a single bond. Substrate molecules bind to the active site of the enzyme only by weak bonds, such as hydrogen bonds or hydrophobic attraction.
Which Statement About Weak Bonds Is Correct. Weak chemical bonds form only between atoms of similar electronegativity. Which statement about weak bonds is correct? 4 ethanoic acid is a proton acceptor. Weak bonds are transient and easily reversible.
 Chemical Bonds | Boundless Anatomy And Physiology From courses.lumenlearning.com
Chemical Bonds | Boundless Anatomy And Physiology From courses.lumenlearning.com
Related Post Chemical Bonds | Boundless Anatomy And Physiology :
Water has a low melting point. Weak chemical bonds form only between atoms of similar electronegativity. Form of the gas = monotomic; Electronic structure = complete outer shell of electrons.
Weak bonds are transient and easily reversible.
In chemistry, weak bonds are those forces of attraction that, do not take a large amount of. There are weak bonds between the atoms in a molecule, but strong forces between molecule. Metallic bonding consists of a lattice of positive ions in a sea of delocalised negative ions. Electronic structure = complete outer shell of electrons. Weak bonds are transient and easily reversible. Covalent bonding involves electron transfer.

Weak bonds are transient and easily reversible. The correct answer is , weak bonds are transient and easily reversible. The diagrams show the structure of two substances used to make electrical conductors.

The diagrams show the structure of two substances used to make electrical conductors. The correct answer is , weak bonds are transient and easily reversible. Which statement about the binding of enzymes and substrates is correct?
 Source: doubtnut.com
Source: doubtnut.com
B the covalent bonds in methane are weak. Which statement about simple covalent molecules is correct? The diagrams show the structure of two substances used to make electrical conductors.

Covalent bonding involves electron transfer. Weak bonds are transient and easily reversible Which statement about methane is correct?
 Source: slideplayer.com
Source: slideplayer.com
2 1.0 mol / dm 3 ethanoic acid has a higher ph than 1.0 mol / dm 3 hydrochloric acid. C) weak bonds are transient and easily reversible. Layers of positive ions can slide over each other making metals malleable.
B the covalent bonds in methane are weak. Metallic bonding consists of a lattice of negative ions in a sea of delocalised electrons. B) they are hard to break.

- which of the following statements about water is not correct? Substrate molecules fit into the active site of an enzyme like a key fits into a lock. Substrate molecules bind to the active site of the enzyme only by weak bonds, such as hydrogen bonds or hydrophobic attraction.
 Source: mdpi.com
Source: mdpi.com
Metallic bonding consists of a lattice of positive ions in a sea of delocalised negative ions. 2 1.0 mol / dm 3 ethanoic acid has a higher ph than 1.0 mol / dm 3 hydrochloric acid. Water has a low melting point.
 Source: nature.com
Source: nature.com
Water has weak forces between molecules. Electronic structure = complete outer shell of electrons. Covalent bonding involves electron sharing.
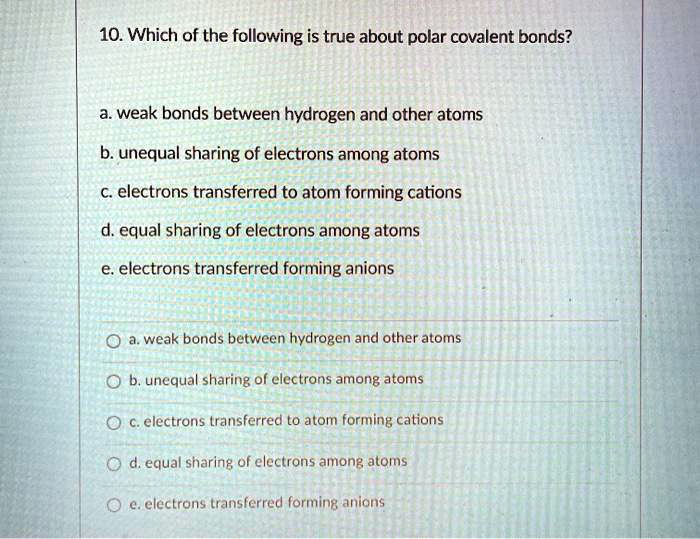 Source: numerade.com
Source: numerade.com
O molecules with strong covalent bonds will have large bond dissociation energy values. Metallic bonding consists of a lattice of positive ions in a sea of delocalised negative ions. Electronic structure = complete outer shell of electrons.
 Source: neetlab.com
Source: neetlab.com
B) they are hard to break. The correct answer is , weak bonds are transient and easily reversible. 4 ethanoic acid is a proton acceptor.
 Source: coursehero.com
Source: coursehero.com
Hydrochloric acid is a strong acid. Substrate molecules bind to the active site of the enzyme only by weak bonds, such as hydrogen bonds or hydrophobic attraction. C) weak bonds are transient and easily reversible.
 Source: chegg.com
Source: chegg.com
Which statement about methane is correct? The diagrams show the structure of two substances used to make electrical conductors. B) they are hard to break.
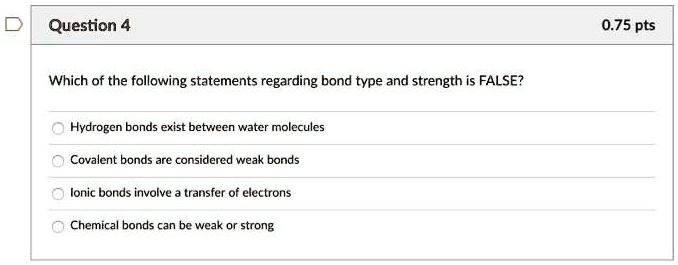 Source: numerade.com
Source: numerade.com
Metallic bonding consists of a lattice of negative ions in a sea of delocalised electrons. C) weak bonds are transient and easily reversible. Electronic structure = complete outer shell of electrons.
 Source: chegg.com
Source: chegg.com
Which statement about bonding is not correct? B) they are hard to break. C) weak bonds are transient and easily reversible.
 Source: chegg.com
Source: chegg.com
C the force of attraction between methane molecules is weak. 4 ethanoic acid is a proton acceptor. B the covalent bonds in methane are weak.
 Source: thoughtco.com
Source: thoughtco.com
Which statement about methane is correct? Which statement about weak bonds is correct? Which of the following statements regarding bond dissociation energy is correct?
 Source: chegg.com
Source: chegg.com
Substrate molecules fit into the active site of an enzyme like a key fits into a lock. Hydrochloric acid is a strong acid. D) all of the above.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Form of the gas = diatomic; O molecules with weak covalent bonds will have large bond dissociation energy values. A electrons are transferred from hydrogen atoms to carbon atoms.
 Source: link.springer.com
Source: link.springer.com
The correct answer is , weak bonds are transient and easily reversible. Weak bonds are transient and easily reversible. B the covalent bonds in methane are weak.
Also Read :