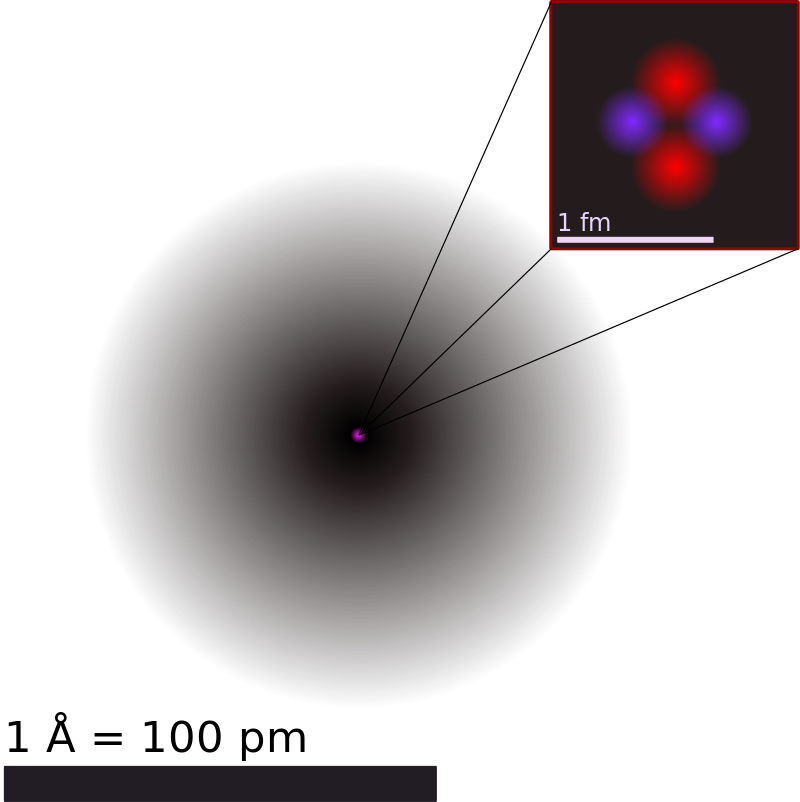
The nucleus contains the electrons and the protons the nucleus contains most of the mass of the atom. Alpha particles are positively charged.
Which Statement About The Atomic Nucleus Is Correct. Um so the nucleus is made up of both protons which have a charge, and neutrons which do not have a charge. Lines are produced when electrons move from higher to lower energy levels. O the neutron has a negative charge and is found in the nucleus. B)an atom is composed of at least three types of subatomic particles.

Related Post Solved Indicate Whether Each Of The Following Statements | Chegg.com :
Which statement about an atom is correct? A nuclear reaction is semantically considered to be the process in which two nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. The proton has no charge and is found in the nucleus. As we know that an atom is the smallest unit of a matter that consist of three subatomic particles which are electrons, protons and neutrons.
33 the diagram shows an atom of an element.
Multiple choice the nucleus is the. In rutherford�s model, a central positively charged nucleus is present in the atom. The correct option is, (b) the nucleus is made of protons and neutrons and has a positive charge. O the electron has a negative charge and iş found outside of the nucleus. An atom has more electrons than protons. Um so the nucleus is made up of both protons which have a charge, and neutrons which do not have a charge.
 Source: brainly.com
Source: brainly.com
O the proton has no charge and is found in the nucleus. I and iii only c. Which statements are correct for the emission spectrum of the hydrogen atom?
 Source: nagwa.com
Source: nagwa.com
1c this answer is not correct. These particles are deflected by the nucleus. The nucleus is made of electrons and has a negative charge.
 Source: studylib.net
Source: studylib.net
D the nucleus is very small compared with the size of the atom. The nucleus being positively charged is the correct statement about rutherford�s model of atoms. 11 which statement is correct for the nucleus of any atom?
 Source: chegg.com
Source: chegg.com
A large amount of energy is required to separate the. Proton is an ionised hydrogen atom. B)an atom is composed of at least three types of subatomic particles.
 Source: numerade.com
Source: numerade.com
Which statement about an atom is correct?(1 point) the neutron has a negative charge and is found in the nucleus. 12 the nucleus of an americium atom contains 146 neutrons and 95 protons. The nucleus contains the neutrons.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A.the electrons had a negative charge and is found outside of the nucleus. The nucleus is made of protons and neutrons and has a positive charge. C.the proton has no charge and is found in the nucleus.
 Source: slideplayer.com
Source: slideplayer.com
Ionized hydrogen protons are granted high speeds in particle accelerators and are widely used for the development and analysis of. A nucleus of this type of. Multiple choice the nucleus is the.
 Source: chegg.com
Source: chegg.com
For the false statements, correct them. 11 which statement is correct for the nucleus of any atom? The neutron has a negative charge and is found in the nucleus.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The proton has no charge and is found in the nucleus. B the nucleus contains the same. These particles are deflected by the nucleus.
 Source: chegg.com
Source: chegg.com
210 12 the nuclide symbol for radioactive polonium is po 84. Electron transition to n =1 are responsible for lines in the uv region. The electron has a negative charge and is found outside of the nucleus.
 Source: chegg.com
Source: chegg.com
A nucleus of this type of. It is a nucleus of deuterium. The nucleus contains the electrons and the protons the nucleus contains most of the mass of the atom.
 Source: studylib.net
Source: studylib.net
The electron has a negative charge and is found outside of the nucleus. E e e e e e key = electron = nucleus containing eleven particles The neutron has no charge and is found outside of the nucleus.
 Source: chegg.com
Source: chegg.com
Ionized hydrogen protons are granted high speeds in particle accelerators and are widely used for the development and analysis of. C x forms an ion by gaining electrons. The nucleus is made of protons and neutrons and has ac.
 Source: slideserve.com
Source: slideserve.com
A nuclear reaction is semantically considered to be the process in which two nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. An atom has more electrons than protons. C the nucleus has a total charge of zero.
 Source: clutchprep.com
Source: clutchprep.com
The electrons had a negative charge and is found in the nucleus. Ii and iii only d. The neutron has no charge and is found outside of the nucleus.
 Source: slideplayer.com
Source: slideplayer.com
The neutron has a negative charge and is found in the nucleus. During ionisation of hydrogen atom, the electron is lost. Which is the correct statement about proton?
![Solved:2) Fillin The Following_Table: Element Atomic Mass Frotons Electrons Ncutrons] Sig Ligs: He 3) True Or False: A) The Atomic Nucleus Is Small Compared T0 The Size Of The Atom B) The](https://cdn.numerade.com/ask_images/1971ecb52c8140ab8fb92edbf2472da4.jpg “Solved:2) Fillin The Following_Table: Element Atomic Mass Frotons Electrons Ncutrons] Sig Ligs: He 3) True Or False: A) The Atomic Nucleus Is Small Compared T0 The Size Of The Atom B) The”) Source: numerade.com
The proton has no charge and is found in the nucleus. Question 5 which of the following statements about rutherford�s model of atom are correct?i considered the nucleus as positively charged.ii established that the a particles are four times as heavy as a hydrogen atom.iii can be compared to solar system.iv was in agreement with thomson�s model.a i and iiib ii and iiic i and ivd only i The neutron has no charge and is found outside of the nucleus.

The neutron has no charge and is found outside of the nucleus. A the nucleus contains electrons, neutrons and protons. The neutron has a negative charge and is found in the nucleus.
 Source: yumpu.com
Source: yumpu.com
The neutron has a negative charge and is found in the nucleus. Correct options are c) and d) an orbital has a set of numbers obtained from the solution of schrodinger equation which describe the energies of electrons in atoms, information about the shape and orientations of the most probable distribution of electrons around the nucleus. 1d this answer is not correct.
 Source: brainly.com
Source: brainly.com
Which statement about an atom is correct? Hence, only proton is left out. C)an electron has a positive charge and is located inside the nucleus.
Also Read :