Paramagnetic species in the following is clo2 only. The os centre is in a +2 oxidation state.
Which Species Is Diamagnetic. Overall 4 species are paramagnetic including no, no 2. Therefore due to absence of you do have a sense of unfair electron as to will be as you will be died magnetic in nature. The electronic configuration of no will be (ii) no 2. Water has no unpaired electrons and is thus diamagnetic.
 Which Of The Following Is Diamagnetic? From toppr.com
Which Of The Following Is Diamagnetic? From toppr.com
Related Post Which Of The Following Is Diamagnetic? :
Which among the following are diamagnetic? We review their content and use your feedback to keep the quality high. The os centre is in a +2 oxidation state. The electronic configuration of no will be (ii) no 2.
The electronic configurations of molecules given in the options are as follows (i) no.
If the atom or molecule has. Therefore due to absence of you do have a sense of unfair electron as to will be as you will be died magnetic in nature. Water has no unpaired electrons and is thus diamagnetic. Hope that we can help you do this homework as exactly as possible. Here the molecule c2 has all paired electrons in its electronic configuration. Overall 4 species are paramagnetic including no, no 2.
 Source: toppr.com
Source: toppr.com
Here the molecule c2 has all paired electrons in its electronic configuration. For diamagnetic behaviour number of unpaired electrons should be zero. All three of the species you wrote here are paramagnetic, assuming the 2 is a subscript in each of the structures.
 Source: brainly.in
Source: brainly.in
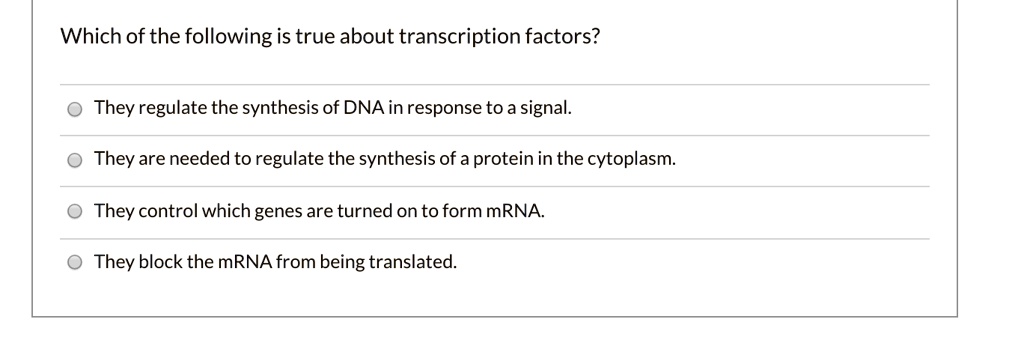
Using mo theory, predict which of the following species is diamagnetic: All electrons can not be paired up and there is an odd electron. Total number of electrons = 7+8+8 = 23.
 Source: numerade.com
Source: numerade.com
Chemical bonding and molecular structure. What is the magnetic property of c2? Overall 4 species are paramagnetic including no, no 2.
 Source: clutchprep.com
Source: clutchprep.com
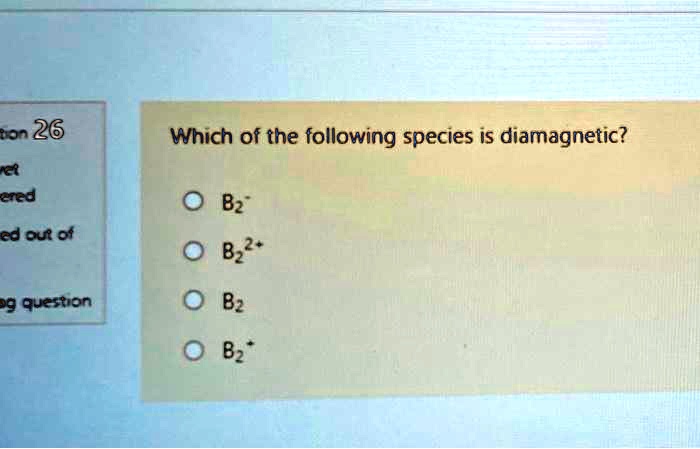
As os is in group 8, os2+ has (8. Which of the following species is diamagnetic? All three of the species you wrote here are paramagnetic, assuming the 2 is a subscript in each of the structures.
 Source: chegg.com
Source: chegg.com
If all the electrons in the atom or molecule are paired then the substance is diamagnetic. And beard electrons present here. So according to the option, option b eat correct answer ups and be it correct answer.
 Source: youtube.com
Source: youtube.com
← prev questionnext question →. Using mo theory, predict which of the following species is diamagnetic: This is the best answer based on feedback and ratings.

If all the electrons in the atom or molecule are paired then the substance is diamagnetic. Asked mar 6, 2018 in class xi chemistry by vijay expert (7.9k points) diamagnetic species are those which contain no unpaired electrons. Chemical bonding and molecular structure.
 Source: toppr.com
Source: toppr.com
Which of the following species is diamagnetic? A species is said to be diamagnetic when it has all the paired electrons. So according to the option, option b eat correct answer ups and be it correct answer.

Experts are tested by chegg as specialists in their subject area. Species like b2 are paramagnetic due to presence of two unpaired electrons in pi 2p bonding molecular orbitals according to molecular orbital theory. The neutral oxygen is paramagnetic according to mo theory because it.
 Source: toppr.com
Source: toppr.com
Using mo theory, predict which of the following species is diamagnetic: We review their content and use your feedback to keep the quality high. Similarly if the species contain unpaired electron it is said to be paramagnetic.
 Source: doubtnut.com
Source: doubtnut.com
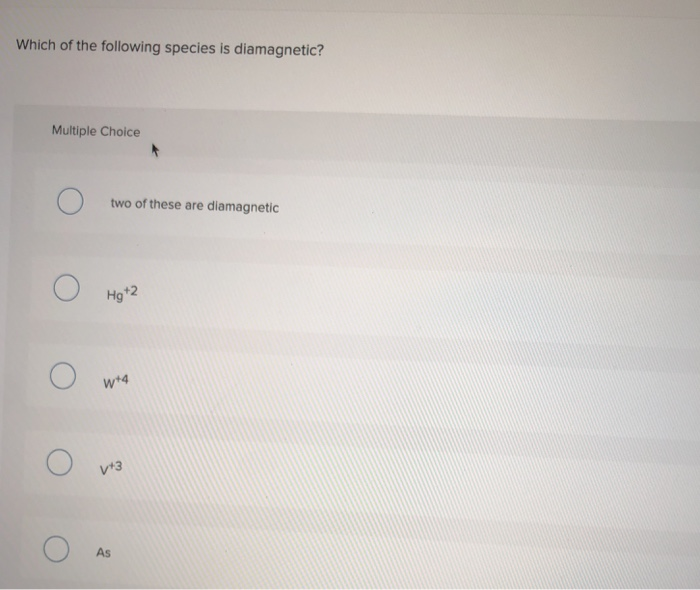
← prev questionnext question →. Diamagnetic species are those species which have paired electrons in their molecular orbitals. What is the magnetic property of c2?
 Source: numerade.com
Source: numerade.com
Therefore the answer is option (d). If the bond order is integer, species is diamagnetic ( except 10 electron, 16 electron species). Since the 4d and 5s subshells are entirely filled, there are no unpaired electrons and sn2+ by definition is diamagnetic.
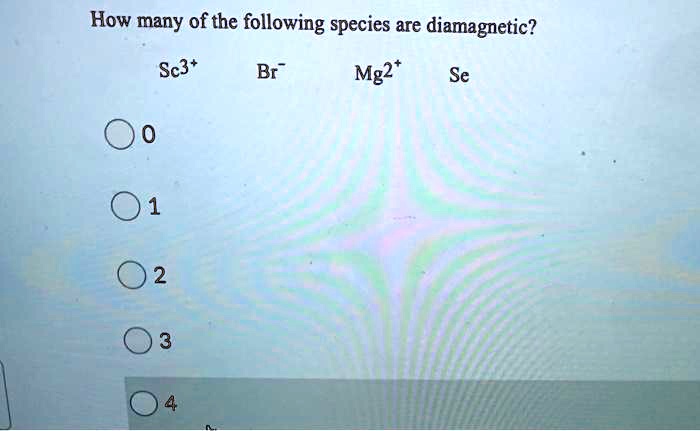 Source: numerade.com
Source: numerade.com
Are filled accordingly whereas in the case of molecules which consists of minimum two atoms we will be dealing with molecular. Are filled accordingly whereas in the case of molecules which consists of minimum two atoms we will be dealing with molecular. So according to the option, option b eat correct answer ups and be it correct answer.

Species like b2 are paramagnetic due to presence of two unpaired electrons in pi 2p bonding molecular orbitals according to molecular orbital theory. If all the molecular orbitals in a molecule are doubly occupied, the substance is diamagnetic. Asked mar 6, 2018 in class xi chemistry by vijay expert (7.9k points) diamagnetic species are those which contain no unpaired electrons.
 Source: brainly.in
Source: brainly.in
The electronic configurations of molecules given in the options are as follows (i) no. On page 71 of tbr chem, it states this about diamagnetic species: In this problem, i can write the expression of as to add sigma one h two, seeing my start one is zero.
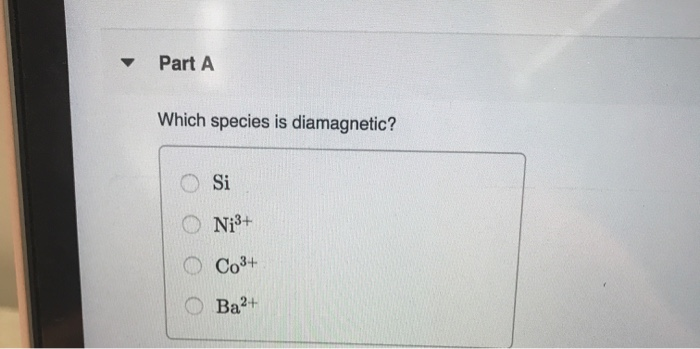 Source: chegg.com
Source: chegg.com
A species is said to be diamagnetic when it has all the paired electrons. So according to the option, option b eat correct answer ups and be it correct answer. Total number of electrons = 7+8 = 15.
 Source: toppr.com
Source: toppr.com
What is the magnetic property of c2? Paramagnetic species in the following is clo2 only. All electrons can be paired up and there is no free electron.
 Source: clutchprep.com
Source: clutchprep.com
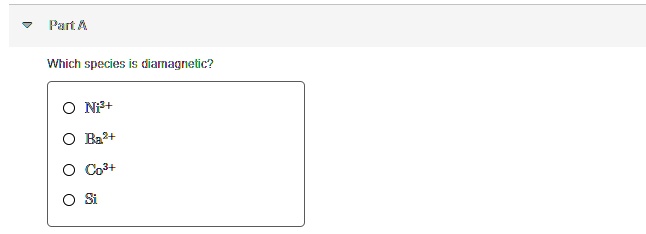
Chemical bonding and molecular structure. The electronic configuration of no 2 will be Which of the following species is diamagnetic?

Generally, all materials have the diamagnetic properties, making a weak contribution to the magnetic behaviour of the material when subjected to an external magnetic field. Chemical bonding and molecular structure. Species like b2 are paramagnetic due to presence of two unpaired electrons in pi 2p bonding molecular orbitals according to molecular orbital theory.
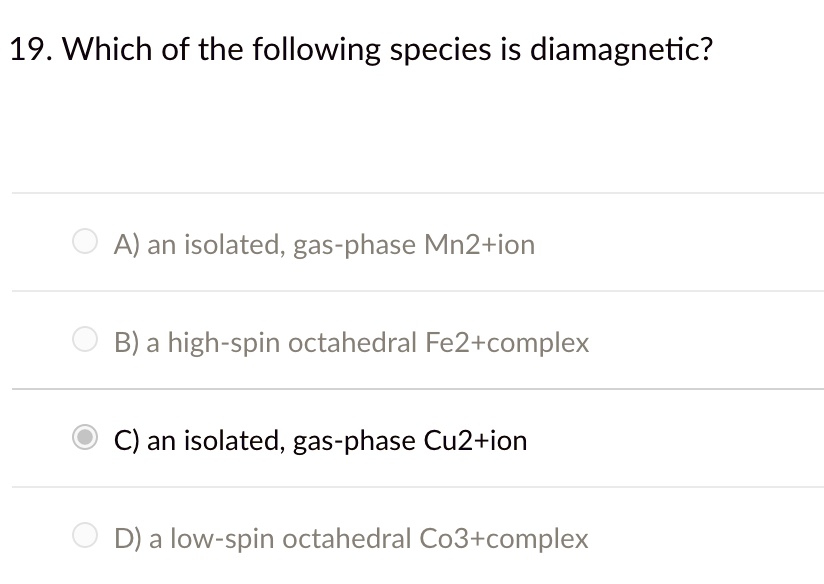 Source: itprospt.com
Source: itprospt.com
As os is in group 8, os2+ has (8. What is the magnetic property of c2? And beard electrons present here.
Also Read :