Who wrote the first modern chemical textbook? On the basis of this observation, newland’s law of octaves was formulated.
Which Scientist Established The Law Of Octaves. He found that every eight elements had similar properties and called this the law of octaves. He arranged them in an ascending order based on their atomic masses and observed that every 8th element had similar properties. The pattern showed that each element was similar to the element eight places ahead of it. In the late 1800s, john newlands put the known elements at.
 Newlands Law Octaves Table Stock Illustration 1669314496 From shutterstock.com
Newlands Law Octaves Table Stock Illustration 1669314496 From shutterstock.com
Related Post Newlands Law Octaves Table Stock Illustration 1669314496 :
Newlands studied at the royal college. In chemistry, the law of octaves was proposed by the english chemist j.a.r. Who wrote the first modern chemical textbook? Law of octaves states that if the chemical elements are arranged according to increasing atomic weight, those with similar physical and chemical properties occur after each interval of seven elements.
In the year 1864, the british chemist john newlands attempted the 62 elements known at that time.
The correct answer is john alexander reina newlands. Newlands in 1865 that, if the chemical elements are arranged according to increasing atomic weight, those with similar physical and chemical properties occur after each interval of seven elements.newlands was one of the first to detect a periodic pattern in the properties of the. The law of octaves was given by john newlands. Which scientist established the law of octaves? Was asked on may 31 2017. It states the every eighth element will be similar to every first element.
 Source:
Source:
The law of octaves was given by john newlands. In chemistry, the law of octaves was proposed by the english chemist j.a.r. An english scientist called john newlands put forward his law of octaves in 1864.
 Source: toppr.com
Source: toppr.com
Was asked on may 31 2017. True false valence electrons are also known as the innermost electrons. In , john newlands established known elements in increasing order of their atomic mass.
 Source: edusaint.com
Source: edusaint.com
Newlands studied at the royal college. In chemistry, the law of octaves was proposed by the english chemist j.a.r. He arranged them in an ascending order based on their atomic masses and observed that every 8th element had similar properties.
 Source: youtube.com
Source: youtube.com
When he did this, he found that each element was similar to the element eight places further on. In , john newlands established known elements in increasing order of their atomic mass. It states the every eighth element will be similar to every first element.
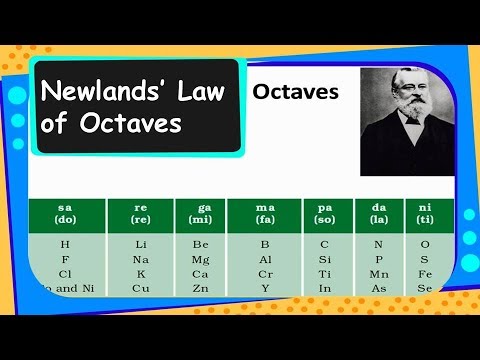 Source: youtube.com
Source: youtube.com
According to gurdjieff, in order to understand this law, it is first necessary to regard the universe as consisting of vibrations. Who wrote the first modern chemical textbook? Law of octaves states that if the chemical elements are arranged according to increasing atomic weight, those with similar physical and chemical properties occur after each interval of seven elements. that is every 8th element have the same physical and chemical properties.
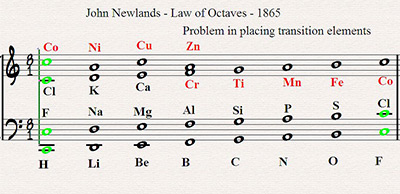
Law of octaves states that if the chemical elements are arranged according to increasing atomic weight, those with similar physical and chemical properties occur after each interval of seven elements. Newlands in 1865 that, if the chemical elements are arranged according to increasing atomic weight, those with similar physical and chemical properties occur after each interval of seven elements.newlands was one of the first to detect a periodic pattern in the properties of the. Check the discoveries of each scientist, to know who has given this law.
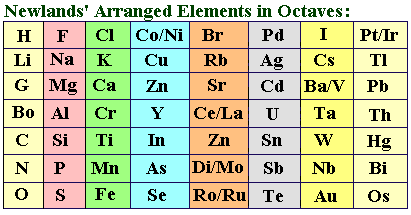 Source: periodtables.blogspot.com
Source: periodtables.blogspot.com
In the year 1864, the british chemist john newlands attempted the 62 elements known at that time. The correct answer is john alexander reina newlands. John newlands, in full john alexander reina newlands, (born november 26, 1837, london, england—died july 29, 1898, london), english chemist whose “law of octaves” noted a pattern in the atomic structure of elements with similar chemical properties and contributed in a significant way to the development of the periodic law.
 Source: brainly.com
Source: brainly.com
An english scientist called john newlands put forward his law of octaves in 1864. This set is often in folders with. It states the every eighth element will be similar to every first element.
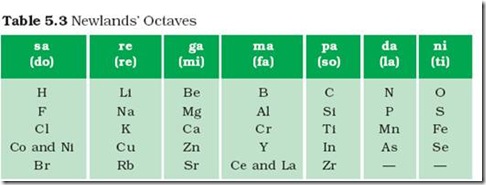 Source: cbse.myindialist.com
Source: cbse.myindialist.com
The law failed because of the. The correct answer is john alexander reina newlands. Which scientist established the law of octaves?
 Source: study.com
Source: study.com
Law of octaves, in chemistry, the generalization made by the english chemist j.a.r. He arranged them in ascending order according to their atomic weights and discovered that the properties of every eighth element were the same. What is modern period law?
 Source: wired.com
Source: wired.com
According to gurdjieff, in order to understand this law, it is first necessary to regard the universe as consisting of vibrations. An english scientist called john newlands put forward his law of octaves in 1864. Which scientist established the law of octaves?
 Source: shutterstock.com
Source: shutterstock.com
In the law of octaves, the first element and the eighth element are related to each other in properties. By arranging the elements according to atomic number instead of atomic mass. It states the every eighth element will be similar to every first element.
 Source: youtube.com
Source: youtube.com
The pattern showed that each element was similar to the element eight places ahead of it. Newlands in 1865 that, if the chemical elements are arranged according to increasing atomic weight, those with similar physical and chemical properties occur after each interval of seven elements.newlands was one of the first to detect a periodic pattern in the properties of the. The law failed because of the.
 Source: brainly.com
Source: brainly.com
He established the law of octaves which basically set the elements according to their atomic number and grouped them according to their physical and chemical properties. It is based on seven musical notes, which are do, re, mi, fa, so, la, ti. In , john newlands established known elements in increasing order of their atomic mass.
 Source: sciencenotes.org
Source: sciencenotes.org
Newlands noted that many pairs of similar elements existed which differed by some multiple of eight in atomic number. According to gurdjieff, in order to understand this law, it is first necessary to regard the universe as consisting of vibrations. The correct answer is john alexander reina newlands.
 Source: moviecultists.com
Source: moviecultists.com
It states the every eighth element will be similar to every first element. He found that every eight elements had similar properties and called this the law of octaves. True false henry moseley proposed the law of octaves in which he explains that the atomic masses of the elements when arranged in ascending order, elements falling in every eight position were deemed to have the same properties.

In the law of octaves, the first element and the eighth element are related to each other in properties. According to gurdjieff, in order to understand this law, it is first necessary to regard the universe as consisting of vibrations. Was asked on may 31 2017.
 Source: edurev.in
Source: edurev.in
This set is often in folders with. In chemistry, the law of octaves was proposed by the english chemist j.a.r. Why did the law of octaves fail?
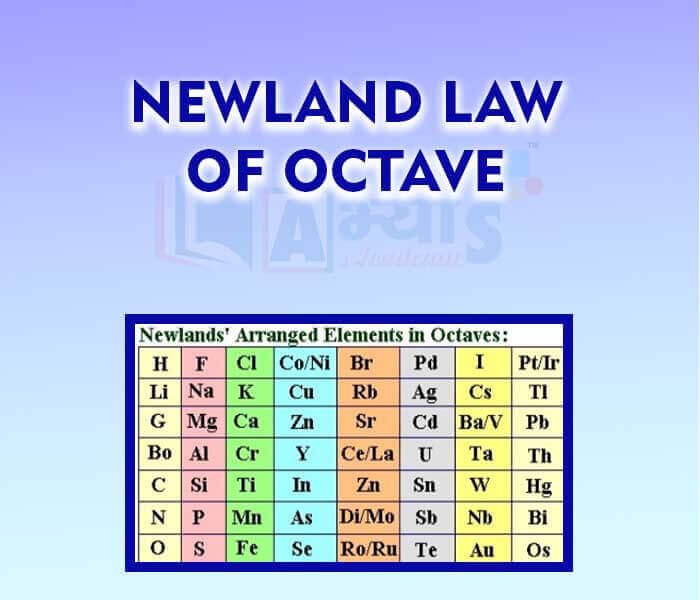 Source: abhyasonline.in
Source: abhyasonline.in
True false henry moseley proposed the law of octaves in which he explains that the atomic masses of the elements when arranged in ascending order, elements falling in every eight position were deemed to have the same properties. The term octaves means the interval between the two musical notes, the first note and the eighth note. By arranging the elements according to atomic number instead of atomic mass.
 Source: slideplayer.com
Source: slideplayer.com
An english scientist called john newlands put forward his law of octaves in 1864. He arranged them in ascending order according to their atomic weights and discovered that the properties of every eighth element were the same. Law of octaves states that if the chemical elements are arranged according to increasing atomic weight, those with similar physical and chemical properties occur after each interval of seven elements. that is every 8th element have the same physical and chemical properties.
Also Read :





