Cesium, gallium, and mercury are the only three metals that are liquid at or around room temperature. Cesium's extraordinary use is that it is utilized in the production of the most exact atomic clock.
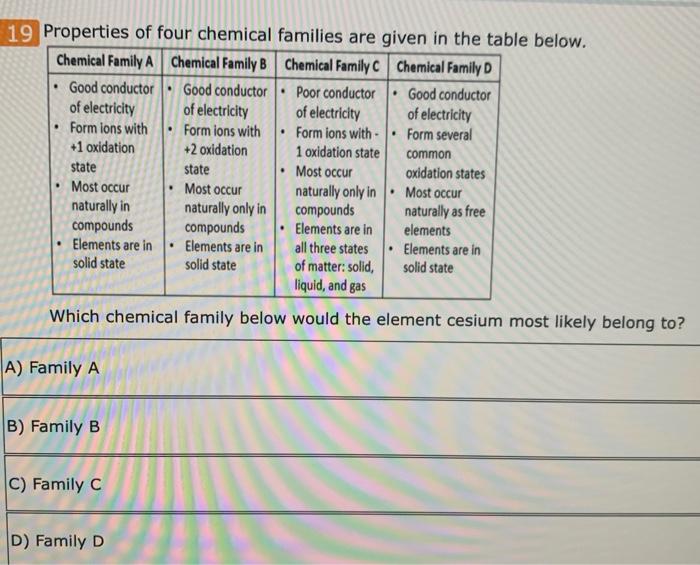
Which Property Would Cesium Most Likely Have. Which of the following elements is most likely to have similar properties to those of sodium (na)? It is the most electropositive element. From the property of k. Cesium, gallium, and mercury are the only three metals that are liquid at or around room temperature.
 What Is The Most Reactive Metal On The Periodic Table? From thoughtco.com
What Is The Most Reactive Metal On The Periodic Table? From thoughtco.com
Related Post What Is The Most Reactive Metal On The Periodic Table? :
Which element is most likely to have six electrons in its 4th energy level? The alkali metals show similar qualities to sodium as it is too. Alkali metals have one electron in the outermost shell. Therefore, we can conclude that a property cesium would most.
Which element is most likely to have six electrons in its 4th energy level?
Namely, alkali metals are lithium, sodium, potassium. He instructs them to choose elements from the four shaded portions of the periodic table described by the diagram. What properties does cesium have? Which of the following elements is most likely to have similar properties to those of sodium (na)? It is the lightest of the metals, with a density approximately half that of water. Cesium is used in atomic clocks and more recently in ion propulsion systems.
 Source: brainly.com
Source: brainly.com
Alloys of alkali metals exist that melt as low as −78 °c (−109 °f). The atoms of which element in group 16 (via) of the periodic table have the greatest tendency to gain electrons? What properties does cesium have?
 Source: en.wikipedia.org
Source: en.wikipedia.org
Cesium salts are used to strenght various types of glass. Has the most metallic character? Magnesium (mg) sulfur (s) francium (fr).

Namely, alkali metals are lithium, sodium, potassium. Namely, alkali metals are lithium, sodium, potassium. Group 1a elements are highly reactive in nature.
Cesium is utilized in producing optical glasses and other optical instruments. Namely, alkali metals are lithium, sodium, potassium. Cesium salts are used to strenght various types of glass.
 Source: mdpi.com
Source: mdpi.com
Lithium, beryllium, and boron nitrogen, arsenic, and antimony potassium, calcium, and gallium cesium, platinum, and radon nitrogen, arsenic, and antimony is the group containing elements that are most likely to have similar properties. Based on the research of albert einstein, what change would most likely result in stopping the emission of electrons from this metal? Gomez has asked his students to choose two elements from the periodic table which are most similar to each other.
 Source: britannica.com
Source: britannica.com
Cesium is described by the german institute for strategic metals (ise) as “the most electropositive of all stable elements in the periodic table”, and the heaviest of the stable metals. Magnesium (mg) sulfur (s) francium (fr). When a gas changes back into a liquid, it is called _____.condensation vaporization sublimation the freezing point user:
 Source: britannica.com
Source: britannica.com
Cesium, gallium, and mercury are the only three metals that are liquid at or around room temperature. The answer is that it is ductile. Element x is in group 2 (iia) and element y is in group 17 (viia).
 Source: britannica.com
Source: britannica.com
Four students� responses are provided here. Cesium is used in atomic clocks and more recently in ion propulsion systems. The answer is that it is ductile.
 Source: brainly.com
Source: brainly.com
Caesium has physical and chemical properties similar to those of rubidium. You can compare fr vs cs on more than 90 properties like electronegativity , oxidation state, atomic shells, orbital structure, electronaffinity, physical states, electrical conductivity and many more. Also, cesium is not a gas.
 Source: brainly.com
Source: brainly.com
Gomez has asked his students to choose two elements from the periodic table which are most similar to each other. A compound formed between these two elements is most likely to have the formula a. Cesium salts are used to strenght various types of glass.
 Source: brainly.com
Source: brainly.com
The alkali metals show similar qualities to sodium as it is too. The atoms of which element in group 16 (via) of the periodic table have the greatest tendency to gain electrons? It is also known as a cesium clock.

So its property is similar to those like na and k. Which of the following elements is most likely to have similar properties to those of sodium (na)? The answer is that it is ductile.
 Source: quizlet.com
Source: quizlet.com
They are also used to make special optical glass, as a catalyst promoter, in vacuum tubes and in radiation monitoring equipment. A compound formed between these two elements is most likely to have the formula a. What properties does cesium have?
 Source: chegg.com
Source: chegg.com
Cesium is described by the german institute for strategic metals (ise) as “the most electropositive of all stable elements in the periodic table”, and the heaviest of the stable metals. Has the most metallic character? Therefore, we can conclude that a property cesium would most.

It is the most electropositive element. So its property is similar to those like na and k. Which element is most likely to have six electrons in its 4th energy level?
 Source: studylib.net
Source: studylib.net
Alkali metals have one electron in the outermost shell. Which property would cesium most likely have? Cesium is utilized in producing optical glasses and other optical instruments.
 Source: nature.com
Source: nature.com
Namely, alkali metals are lithium, sodium, potassium. Element x is in group 2 (iia) and element y is in group 17 (viia). Hence, cesium is also reactive in nature.
 Source: thoughtco.com
Source: thoughtco.com
The alkali metals show similar qualities to sodium as it is too. Caesium has physical and chemical properties similar to those of rubidium. Cesium is used in atomic clocks and more recently in ion propulsion systems.

See also what does star with circle around it mean. Namely, alkali metals are lithium, sodium, potassium. The alkali metals have low melting points, ranging from a high of 179 °c (354 °f) for lithium to a low of 28.5 °c (83.3 °f) for cesium.
 Source:
Source:
Group 1a elements are highly reactive in nature. The chloride is used in photoelectric cells, in optical instruments, and in increasing the sensitivity of electron tubes. Therefore, we can conclude that a property cesium would most.
Also Read :





