How does the number of valence electrons in atoms of metalloids explain why metalloids are semiconductors? Metalloids are metallic brittle solids.
Which Properties Do Metalloids Share With Metals. They can generally form alloys with metals. Some metalloids such as silicon and germanium become electrical conductors under. They can form alloys with metals. Some metalloids such as silicon and germanium become electrical conductors under special conditions.
 The Difference Between Metals And Nonmetals From thoughtco.com
The Difference Between Metals And Nonmetals From thoughtco.com
Related Post The Difference Between Metals And Nonmetals :
One property of metalloids not shared with metals or nonmetals is that. Metalloids tend to be shiny like metals, but brittle like nonmetals. Some metalloids such as silicon and germanium become electrical conductors under special conditions. Mostmetalloids are semiconductors and thermal conductors.
Both can be pounded into thin sheets.
Metalloids are brittle and also nonmetals are very brittle. They’re also called the semimetals because of the shared properties of these elements along the dividing line between metals and nonmetals. Which property do metalloids share with nonmetals? Metals are also good conductors of electricity. Some metalloids such as silicon and germanium become electrical conductors under special conditions. Other physical properties of metalloids are more variable, including their boiling and melting points, although all metalloids exist as solids at room temperature.
 Source: study.com
Source: study.com
They can generally form alloys with metals. Both can be pounded into thin sheets. Both can react to form acidic compounds.
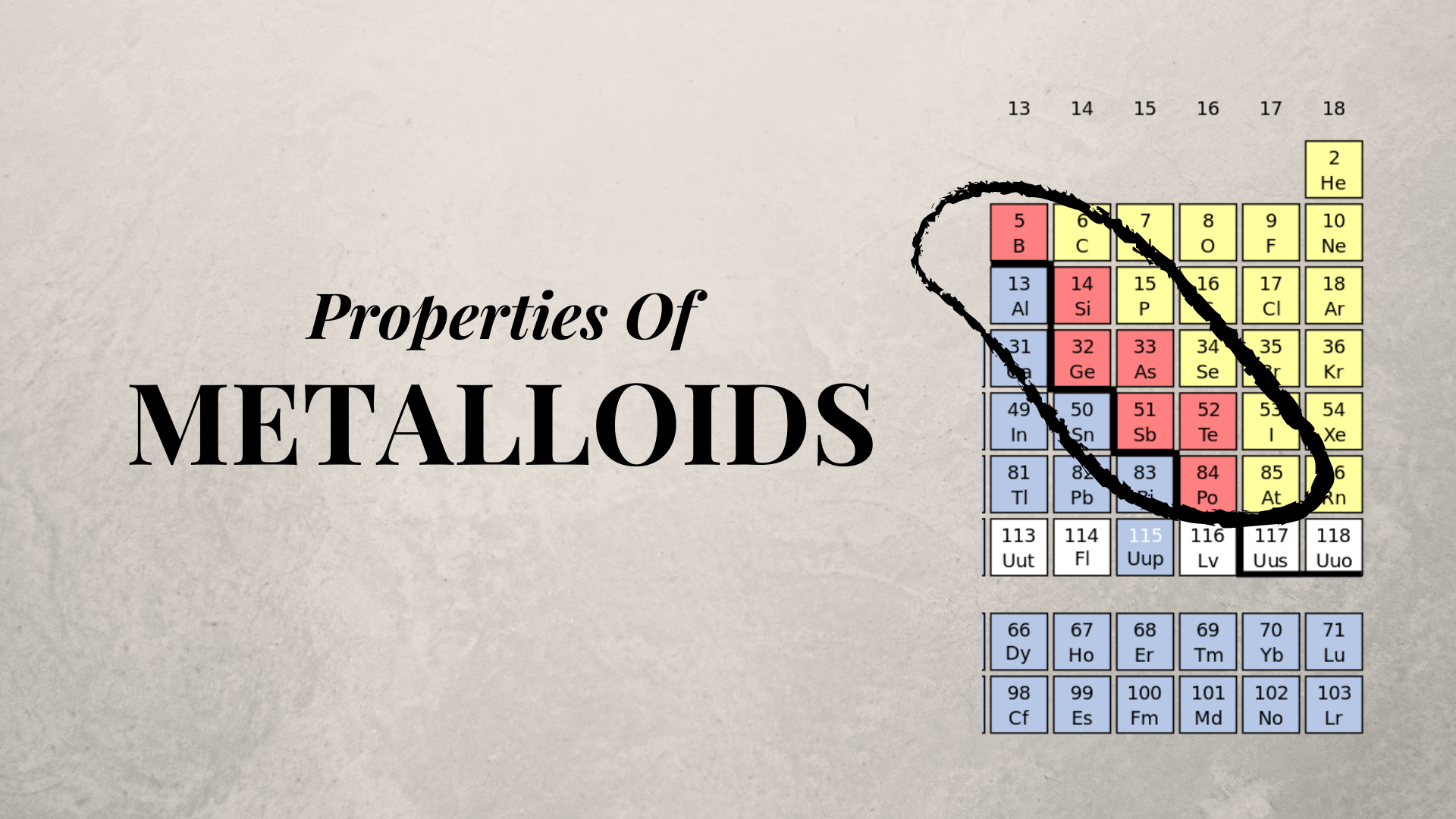 Source: sciencetrends.com
Source: sciencetrends.com
What is its average speed? On the other hand, metalloids are the group of elements that have the properties of metals and nonmetals, these can be both bright and opaque, and their shape can easily change, the metalloids are conductors of heat and electricity, better way. Metalloids have a metallic appearance but are fragile and poor conductors of electricity.
 Source: slideplayer.com
Source: slideplayer.com
Metalloids have a metallic appearance but are fragile and poor conductors of electricity. Both have low melting points. They can generally form alloys with metals.
 Source: pinterest.se
Source: pinterest.se
Both are gases at room temperature. Metalloids can form alloys with other metals, as well as contract during melting. Metalloids are elements which have some intermediate similarities to.
 Source: slidetodoc.com
Source: slidetodoc.com
Metalloids share many similar properties including: They can generally form alloys with metals. Both can be pounded into thin sheets.

Can be hammered into thin sheets. Both can be pounded into thin sheets. Both have low melting points.
 Source: slideplayer.com
Source: slideplayer.com
Metalloids share many similar properties including: One property of metalloids not shared with metals or nonmetals is that. Because they are brittle, they may chip like glass or crumble to a powder if struck.
 Source: slideplayer.com
Source: slideplayer.com
They can generally form alloys with metals. Metalloids are elements which have some intermediate similarities to. Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours.
 Source: mseggleston.com
Source: mseggleston.com
Other physical properties of metalloids are more variable, including their boiling and melting points, although all metalloids exist as solids at room temperature. Metals are generally shiny, malleable, and hard. Metals are also good conductors of electricity.

Metalloids can form alloys with other metals, as well as contract during melting. They have three to six valence electrons, so they can lose or gain electrons. They appear to be metal in appearance, but are brittle.
 Source: chegg.com
Source: chegg.com
Metalloids tend to be shiny like metals, but brittle like nonmetals. Which property do metalloids share with nonmetals? Both are gases at room temperature.
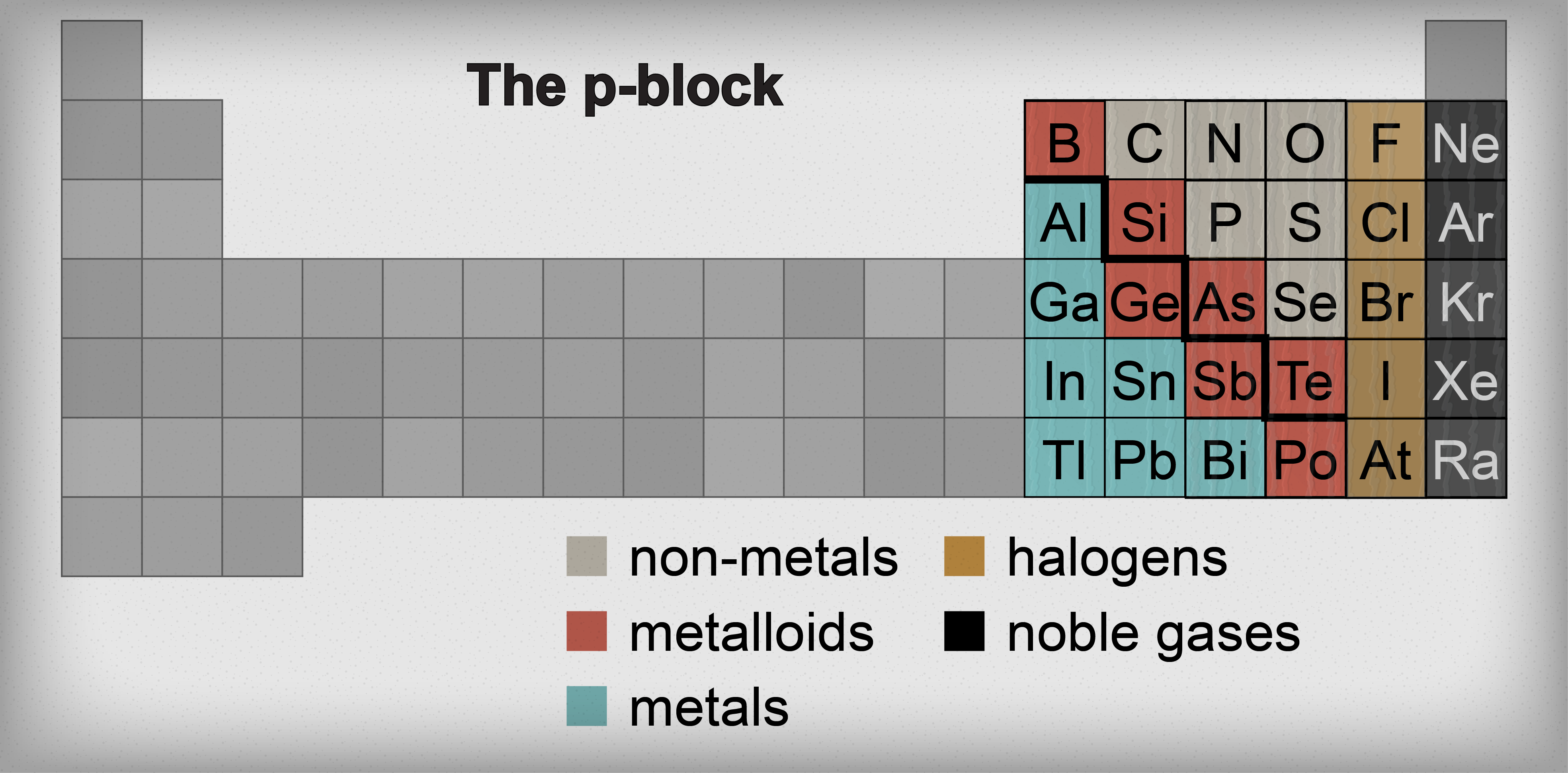 Source: shmoop.com
Source: shmoop.com
Metalloids can form alloys with other metals, as well as contract during melting. They appear to be metal in appearance, but are brittle. Metalloids are elements which have some intermediate similarities to.
 Source: brainly.com
Source: brainly.com
They can generally form alloys with metals. Can be hammered into thin sheets. Both can be pounded into thin sheets.
 Source: slidetodoc.com
Source: slidetodoc.com
They’re also called the semimetals because of the shared properties of these elements along the dividing line between metals and nonmetals. They appear to be metal in appearance, but are brittle. Both are gases at room temperature.
 Source: slideplayer.com
Source: slideplayer.com
On the other hand, metalloids are the group of elements that have the properties of metals and nonmetals, these can be both bright and opaque, and their shape can easily change, the metalloids are conductors of heat and electricity, better way. They can generally form alloys with metals. Metalloids share many similar properties including:

Which of the following properties do metalloids share with nonmetals? Metals are generally shiny, malleable, and hard. They can generally form alloys with metals.
 Source: thoughtco.com
Source: thoughtco.com
The elements are considered metalloids: Is brittle and breaks easily. They can generally form alloys with metals.
 Source: slideplayer.com
Source: slideplayer.com
Metals and non metals.they are neither good or bad conductors of heat. They appear to be metal in appearance, but are brittle. Metalloids have some properties of both metals and nonmetals.
 Source: thoughtco.com
Source: thoughtco.com
Metals are also good conductors of electricity. And electricity and they exist as solids at room temperature. They appear to be metal in appearance, but are brittle.
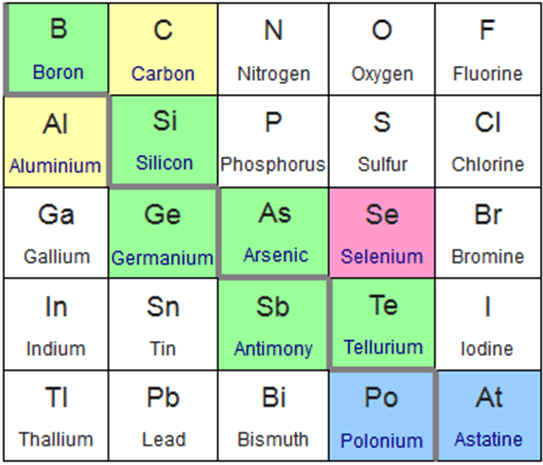 Source: sciencetrends.com
Source: sciencetrends.com
They’re also called the semimetals because of the shared properties of these elements along the dividing line between metals and nonmetals. Both can be pounded into thin sheets. Metalloids are metallic brittle solids.
Also Read :