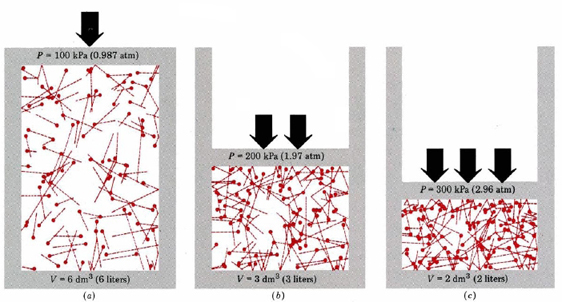
This fact explains the compressibility of gases. Without this we cannot derive any 'real' predictions.
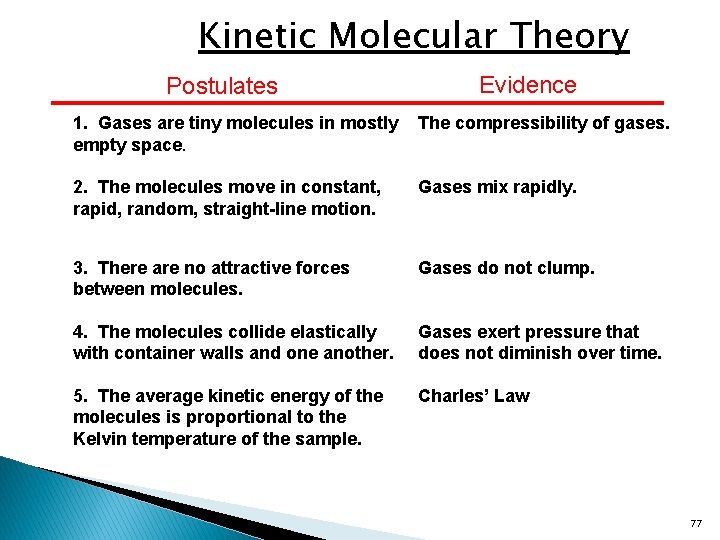
Which Postulate Of The Kinetic Molecular Theory. The molecules are in a continuous random motion, occupy space, and interact with each other through constant collision. As applied to gases, the kinetic molecular theory has the following postulates: So this question asks what postulate of the kinetic molecular theory breaks down under low temperatures. I would say that the main postulate for this theory is statistical mechanics.
 Unit 6 Gases And The Kinetic Molecular Theory From slidetodoc.com
Unit 6 Gases And The Kinetic Molecular Theory From slidetodoc.com
Related Post Unit 6 Gases And The Kinetic Molecular Theory :
One of them is the part that says that there are no attractions between molecules. Hi problem number 30 wants us to discuss which postulates of the kinetic theory for gases are not actually true. So at low temperatures, the postulate that breaks down is that the forces between the gas particles are not significant. Which of the following is included as a postulate in the kinetic molecular theory of an ideal gas?
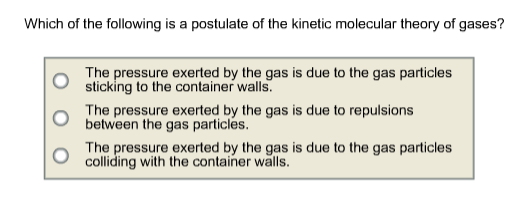
Gas pressure is due to the molecules colliding to the walls of the container.
How does kinetic molecular theory explain gas. (2) gases are composed of molecules whose size is negligible compared to the average distance between them. The kinetic molecular theory of matter states that: Which of the following is a postulate of the kinetic molecular theory of gases? 3 postulates of the kinetic molecular theory: These particles are so small compared to the distances between them that the volume of the individual particles can be assumed to be zero.
 Source: slideserve.com
Source: slideserve.com
Gas pressure is due to the molecules colliding to the walls of the container. I would say that the main postulate for this theory is statistical mechanics. Asked jun 23, 2017 in chemistry by debiwebi.
 Source: quora.com
Source: quora.com
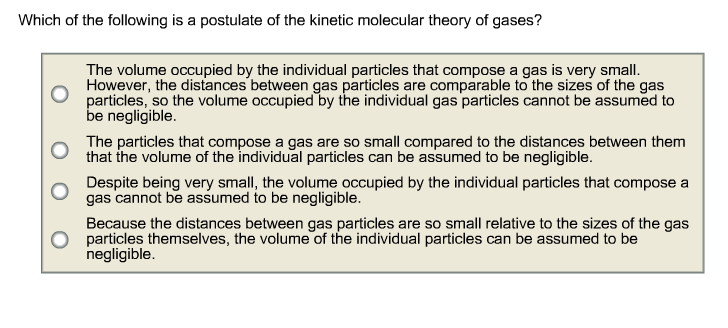
The kinetic molecular theory and graham�s laws the kinetic molecular theory can be used to explain the results graham obtained when he studied the diffusion and effusion of gases. Which of the following is a postulate of the kinetic molecular theory of gases?a. However, the distances between gas particles are comparable to the sizes of the gas particles, so the volume occupied by the individual gas particles cannot be assumed to be negligible.b.
 Source: slideserve.com
Source: slideserve.com
Gases consist of tiny particles (atoms of molecules) postulate 2. The theory is most often applied to gases but is helpful in explaining molecular behavior in all states of matter. All particles have energy, but the energy varies depending on the temperature the sample of matter is in.
 Source: slidetodoc.com
Source: slidetodoc.com
Gases are composed of very tiny particles (molecules). Kinetic molecular theory can be used to explain both charles’ and boyle’s laws. The theory assumes that gases consist of widely separated molecules of negligible volume that are in constant motion, colliding elastically with one another and the walls of their container with average velocities determined by their absolute.
 Source: unacademy.com
Source: unacademy.com
The kinetic molecular theory of gases has the following postulates: 3 postulates of the kinetic molecular theory: Lower kinetic energy reduces successful collisions and thus reduces reactivity and the number of interactions.
 Source: slideplayer.com
Source: slideplayer.com
This in turn determines whether the substance exists in the solid, liquid, or gaseous state. All particles have energy, but the energy varies depending on the temperature the sample of matter is in. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior.

Gases are composed of very tiny particles (molecules). The key to this explanation is the last postulate of the kinetic theory, which assumes that the temperature of a system is proportional to the average kinetic energy of its particles and nothing else. Gases are composed of very tiny particles (molecules).
 Source: chegg.com
Source: chegg.com
All directions of motion for a molecule are equally probable. Lower kinetic energy reduces successful collisions and thus reduces reactivity and the number of interactions. As applied to gases, the kinetic molecular theory has the following postulates:
 Source: youtube.com
Source: youtube.com
Gases are composed of very tiny particles (molecules). The key to this explanation is the last postulate of the kinetic theory, which assumes that the temperature of a system is proportional to the average kinetic energy. The molecules move randomly and their directions only changed after collision with other gas molecules of the walls of the container
 Source: chem.libretexts.org
Source: chem.libretexts.org
So it says that these molecules can move past each other without being. So this question asks what postulate of the kinetic molecular theory breaks down under low temperatures. The kinetic molecular theory of gases has the following postulates:
 Source: slideserve.com
Source: slideserve.com
(2) gases are composed of molecules whose size is negligible compared to the average distance between them. Matter is made up of particles that are constantly moving. The kinetic molecular theory describes the properties of molecules in terms of motion (kinetic energy) and of temperature.
 Source: chegg.com
Source: chegg.com
So at low temperatures, the postulate that breaks down is that the forces between the gas particles are not significant. Which of the following is a postulate of the kinetic molecular theory of gases? How does kinetic molecular theory explain gas.
 Source: slideplayer.com
Source: slideplayer.com
Which postulate or theorem proves that these two triangles are congruent? 2) the average kinetic energy of gases are directly proportional to the absolute temperature in. Using the segment addition postulate, which is true?
 Source: slideplayer.com
Source: slideplayer.com
I would say that the main postulate for this theory is statistical mechanics. This in turn determines whether the substance exists in the solid, liquid, or gaseous state. Um and there are pretty much basically two of those.
 Source: brainly.in
Source: brainly.in
Without this we cannot derive any �real� predictions. Gases consist of tiny particles (atoms of molecules) postulate 2. 1078 students attemted this question.
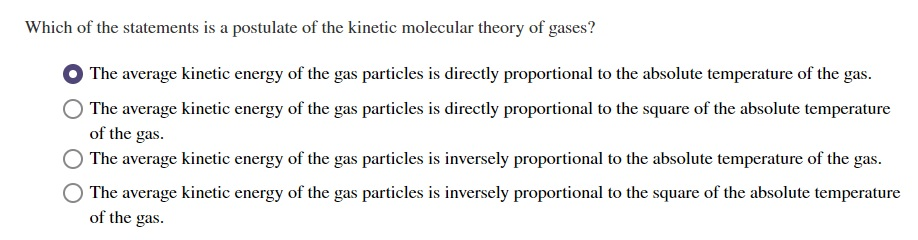 Source: chegg.com
Source: chegg.com
- molecules move in all possible directions with all possible speeds ranging from zero to the speed of light. Gases consist of tiny particles (atoms of molecules) postulate 2. The average kinetic energy of a collection of gas particles is directly proportional to absolute temperature only.
 Source: slideplayer.com
Source: slideplayer.com
As applied to gases, the kinetic molecular theory has the following postulates: The kinetic molecular theory describes the properties of molecules in terms of motion (kinetic energy) and of temperature. Matter is made up of particles that are constantly moving.liquid molecules have more kinetic energy than those.
 Source: unacademy.com
Source: unacademy.com
The kinetic molecular theory is a microscopic model that helps understand what happens to gas particles at the molecular or atomic level when conditions such as. Kinetic molecular theory can be used to explain both charles’ and boyle’s laws. Um and there are pretty much basically two of those.
 Source: co.pinterest.com
Source: co.pinterest.com
The volume occupied by the individual particles that compose a gas is very small. The kinetic molecular theory can be used to explain the results graham obtained when he studied the diffusion and effusion of gases. The kinetic theory of gases refers to the thermodynamic behavior of gases.
 Source: brainly.in
Source: brainly.in
Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. Using the segment addition postulate, which is true? Um and there are pretty much basically two of those.
Also Read :





