Which of the following pairs of elements would be most likely to form an ionic compound? Which pair of elements form an ionic compound?
Which Pairs Of Elements Are Likely To Form Ionic Compounds. Sodium and potassium won�t form an ionic compound. Nonmetals and nonmetals tend to form covalent bonds. Lithium and chlorine and oxygen and bromine are likely to form ionic compound. Which of the following pairs of elements is most likely to form an ionic compound?
 Solved Cu(S) + Hcl(Aq) Cucl2(A) – 23). Mg(S) +- H2(G) | Chegg.com From chegg.com
Solved Cu(S) + Hcl(Aq) Cucl2(A) – 23). Mg(S) +- H2(G) | Chegg.com From chegg.com
Related Post Solved Cu(S) + Hcl(Aq) Cucl2(A) – 23). Mg(S) +- H2(G) | Chegg.com :
Which of the following pairs of elements would be most likely to form an ionic compound? Ionic compounds generally form between elements that are metals and elements that are nonmetals. Which pair of elements is most likely to form an ionic compound? Ionic compounds generally form between elements that are metals and elements that are nonmetals.
A pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal.
The correct pairs of elements likely to form ionic compounds are, potassium and sulfur, magnesium and chlorine. Cl and i, al and k, cl and mg, c and s, al and mg The elements include sulfur, chlorine, nitrogen, fluorine, selenium, oxygen, and iodine. Which of these pairs of elements would be most likely to form an ionic compounds? Ionic compounds allows one or more electrons to be transferred from metals to non metals. Which element would provide one atom to make an ionic bond with calcium?
 Source: chegg.com
Source: chegg.com
Lithium and chlorine will form lithium chloride (licl) oxygen and bromine will form bromine dioxide, because of two electrons from oxygen are transferred. These types of ionic compounds are composed of monatomic cations and. Lithium and chlorine will form lithium chloride (licl) oxygen and bromine will form bromine dioxide, because of two electrons from oxygen are transferred.
 Source: chegg.com
Source: chegg.com
The elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin, bismuth and lead. The models below represent the ionic radii of compounds formed by reacting lithium with. Oppositely changed myst and cr ions athead each and form ionic bond.
 Source: slideplayer.com
Source: slideplayer.com
For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl2). Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. Which of the following pairs of elements is most likely to form an ionic compound?

A pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal. These are well, oxygen is a non metal. Usually, a metallic element and a non metallic element form an ionic compound, but.
 Source: brainly.com
Source: brainly.com
For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl2). The elements include sulfur, chlorine, nitrogen, fluorine, selenium, oxygen, and iodine. You can view more similar questions or.
 Source: chegg.com
Source: chegg.com
Which of the following most likely to form an ionic compound and ionic compounds is formed when we have a metal iron paired up with a nonmetal lion. Which pair of elements is most likely to form an ionic compound? Ionic compounds allows one or more electrons to be transferred from metals to non metals.
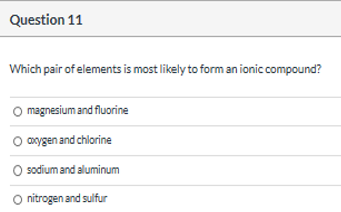 Source: clutchprep.com
Source: clutchprep.com
You can view more similar questions or. K looses one electron to form monoatomic ion; Ionic compounds generally form between elements that are metals and elements that are nonmetals.
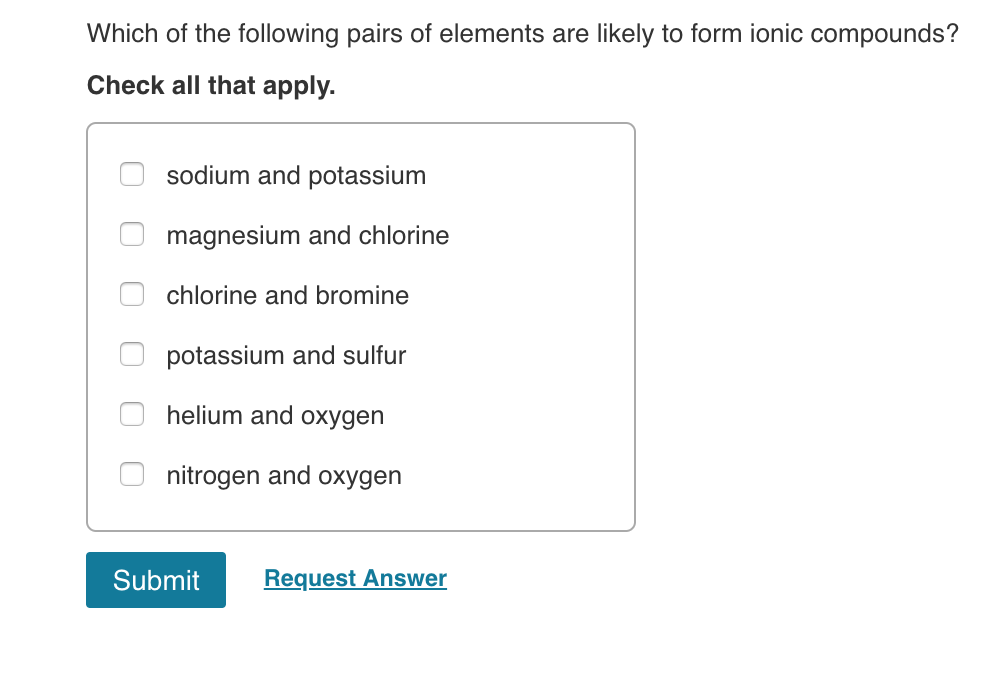 Source: chegg.com
Source: chegg.com
Mg loses two elections to form mght & achieves a complete octet, the el achiere an one election of form cl ions. The elements include sulfur, chlorine, nitrogen, fluorine, selenium, oxygen, and iodine. Which pair of elements form an ionic compound?

Which of the following pairs of elements would be most likely to form an ionic compound? Which of the following pairs of elements is most likely to form an ionic compound? For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl2).
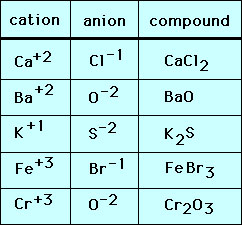 Source: socratic.org
Source: socratic.org
Ionic compounds generally form between elements that are metals and elements that are nonmetals. We have oxygen and roaming. You can view more similar questions or.
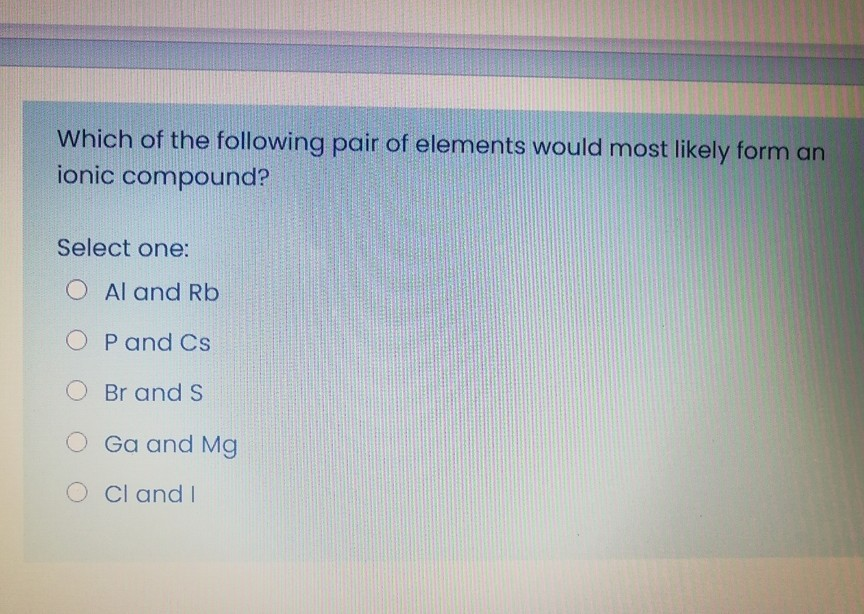 Source: chegg.com
Source: chegg.com
C and o d.al and rb e.o and z Which pair of elements would be most likely to form an ionic compound a. For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl2).
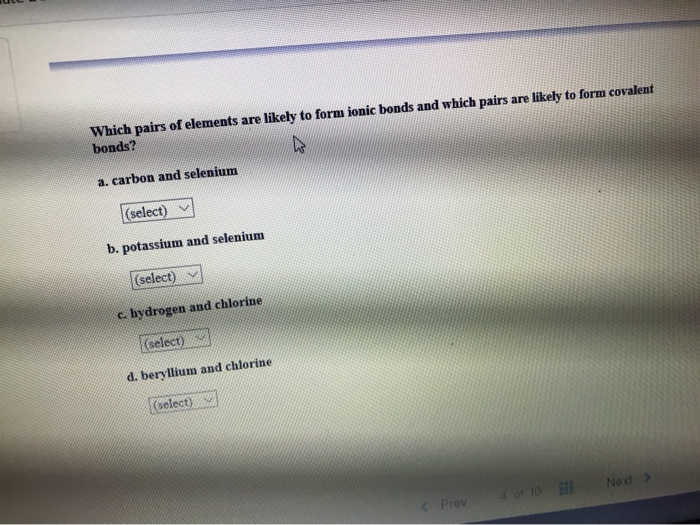 Source: chegg.com
Source: chegg.com
The correct options are the 6 magnesium and chlorine. Therefore, i would say that this would a form mhm and i on a compound for b. Which of the following pairs of elements is most likely to form an ionic compound?
 Source: youtube.com
Source: youtube.com
Which of these pairs of elements would be most likely to form an ionic compounds? Which of these pairs of elements would be most likely to form an ionic compounds? Mg loses two elections to form mght & achieves a complete octet, the el achiere an one election of form cl ions.
 Source: slideplayer.com
Source: slideplayer.com
C and o d.al and rb e.o and z Nonmetals and nonmetals tend to form covalent bonds. So for a we have lithium and chlorine.
 Source: chegg.com
Source: chegg.com
Which of these pairs of elements would be most likely to form an ionic compounds? Which pairs of elements are likely to form ionic compounds? Cl and i, al and k, cl and mg, c and s, al and mg
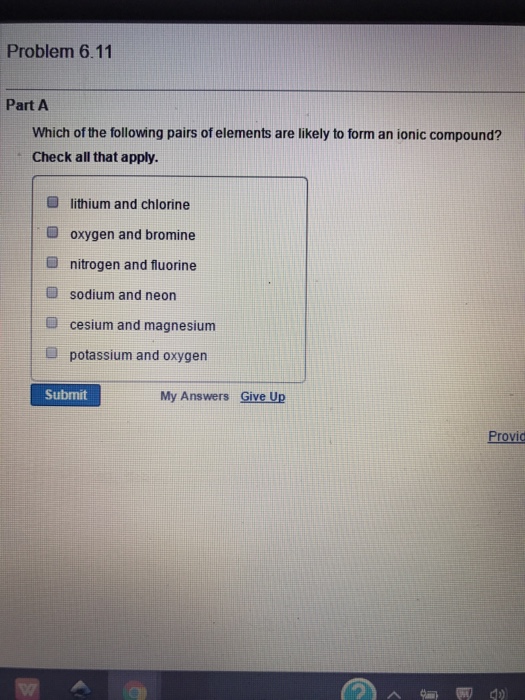
For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl2). Which of these pairs of elements would be most likely to form an ionic compounds? So for a we have lithium and chlorine.
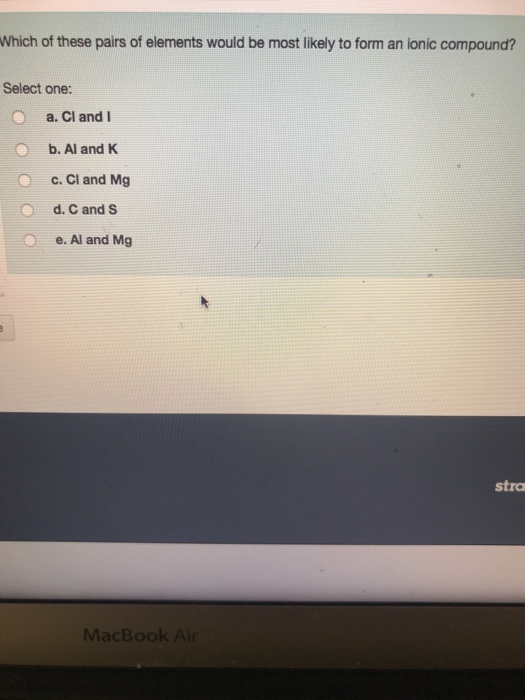
Which pairs of elements are likely to form ionic compounds? For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl2). Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals.
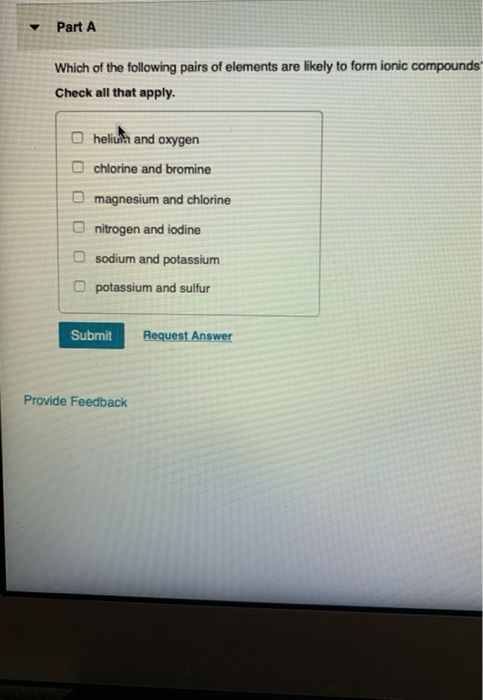 Source: chegg.com
Source: chegg.com
Nonmetals and nonmetals tend to form covalent bonds. Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. Ionic compounds generally form between elements that are metals and elements that are nonmetals.
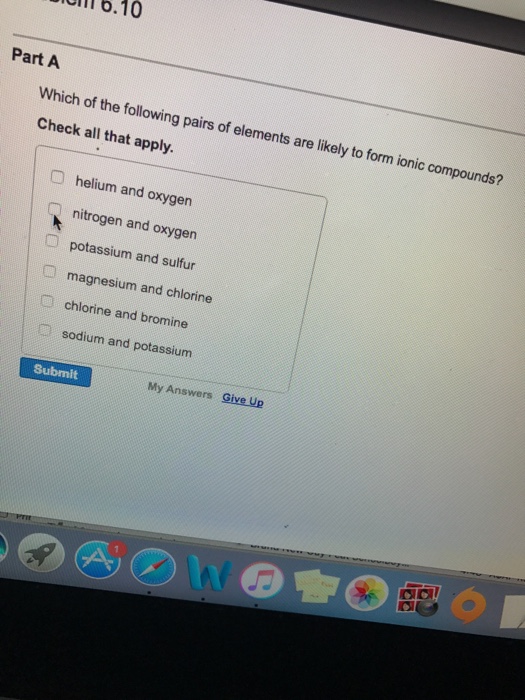
You can view more similar questions or. Potassium and sulfur won�t form an ionic compound. Ionic compounds generally form between elements that are metals and elements that are nonmetals.
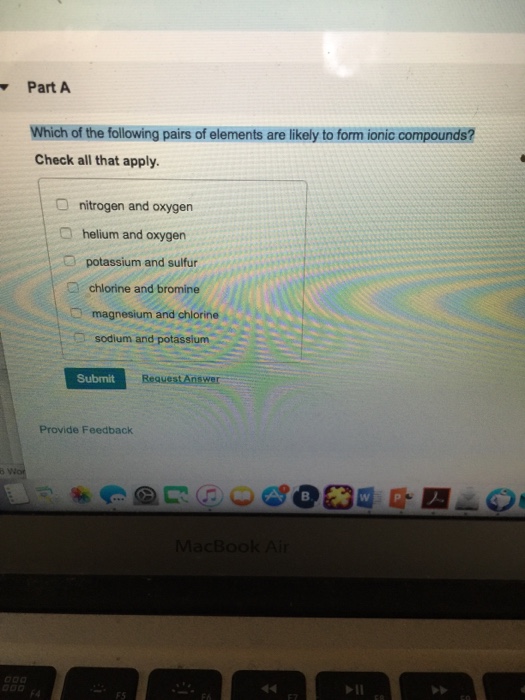
The correct pairs of elements likely to form ionic compounds are, potassium and sulfur, magnesium and chlorine. The elements include sulfur, chlorine, nitrogen, fluorine, selenium, oxygen, and iodine. Usually, a metallic element and a non metallic element form an ionic compound, but.
Also Read :





