Which atom listed has the greatest ability to attract the electrons that form a bond between it and another atom? For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl2).
Which Pair Of Elements Will Form An Ionic Compound. Metals and nonmetals tend to form ionic bonds. Which pair of elements is most likely to kind an ionic compound?sodium and aluminumoxygen and chlorine nitrogen and also sulfurmagnesium and also fluorine magnesium and also flourinemetals and nonmetals often tend to type ionic bonds. Potassium and sulfur won�t form an ionic compound. Which of the following pairs of elements is most likely to form an ionic compound?.
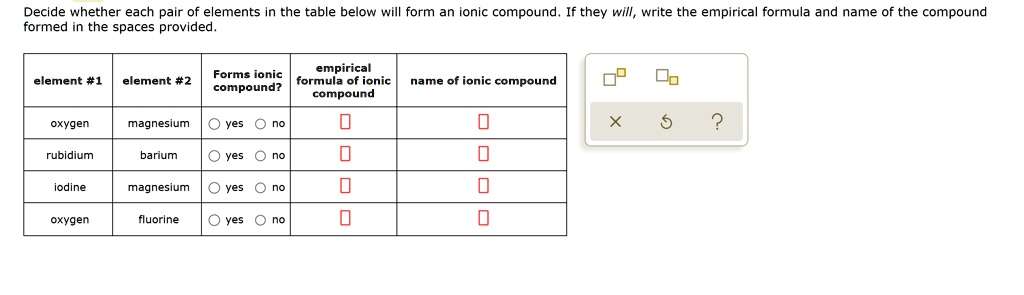 Solved:decide Whether Each Pair Of Elements In The Table Below Will Form An Onic Compound Formed In The Spaces Provided They Will, Write The Empirical Formula And Name Of The Compound Empirical From numerade.com
Solved:decide Whether Each Pair Of Elements In The Table Below Will Form An Onic Compound Formed In The Spaces Provided They Will, Write The Empirical Formula And Name Of The Compound Empirical From numerade.com
Related Post Solved:decide Whether Each Pair Of Elements In The Table Below Will Form An Onic Compound Formed In The Spaces Provided They Will, Write The Empirical Formula And Name Of The Compound Empirical :
Mg is a metal and f is a nonmetal. Let’s imagine what happens when lithium reacts with chlorine to form an ionic compound. But helium is noble gas, which does not form an iron. A binary molecular compound is a molecular compound that is composed of two elements.
Therefore k + and i − ions combine together to form the ionic compound;
In general, the elements that combine to form binary molecular compounds are both nonmetals. Decide whether each pair of elements in the table below will form an ionic compound. A pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal. Does lithium and chlorine form an ionic compound? But helium is noble gas, which does not form an iron. Metals and nonmetals tend to form ionic bonds.
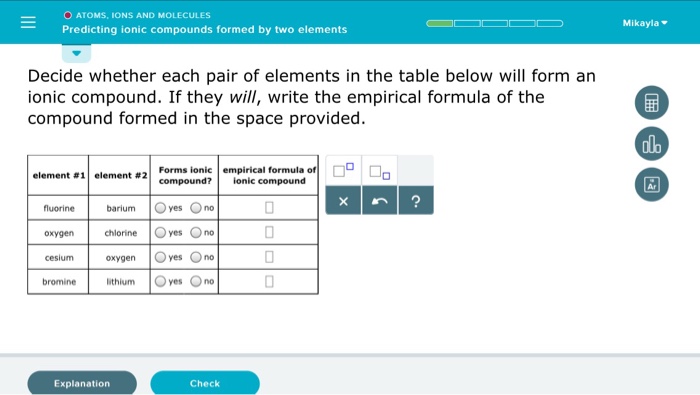
2 📌📌📌 question which of the following pairs of elements would form an ionic compound? For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl 2 ). Simple ionic which one of the following pairs will form an ionic compound with a 1:1 ratio between the cation and anion?
 Source: oneclass.com
Source: oneclass.com
If they will, write the empirical formula and name of the compound formed in the spaces provided. But helium is noble gas, which does not form an iron. Ionic compounds generally form between elements that are metals and elements that are nonmetals.
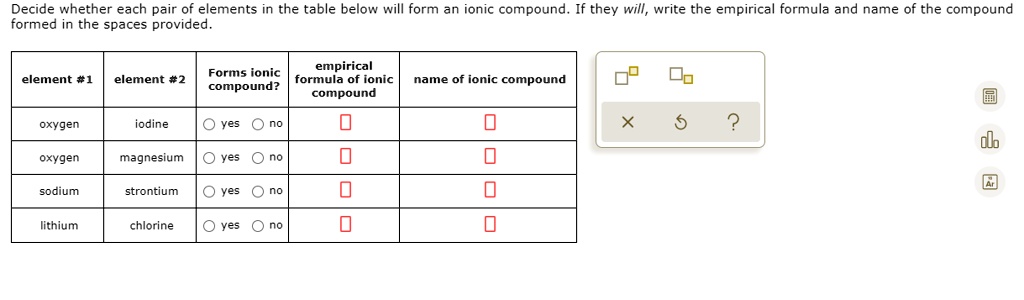 Source: numerade.com
Source: numerade.com
Sulfur loding yes no 0 0 barium cesium yes no 0 An ionic compound has the formula x2o. Simple ionic which one of the following pairs will form an ionic compound with a 1:1 ratio between the cation and anion?
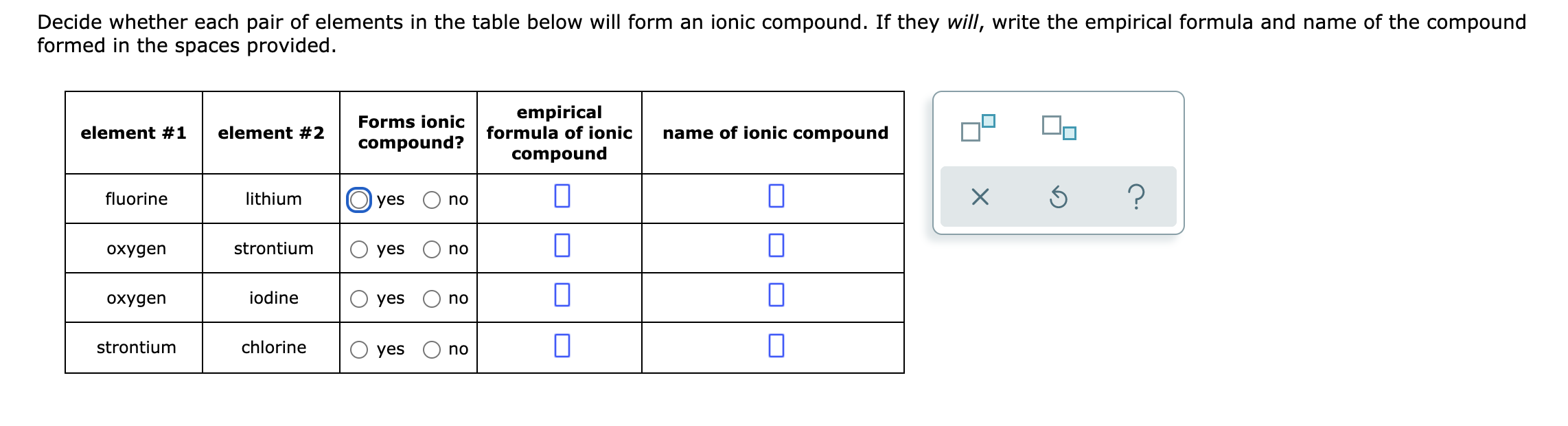 Source: bartleby.com
Source: bartleby.com
A binary molecular compound is a molecular compound that is composed of two elements. The chart below shows monatomic ions formed when an atom loses or gains one or more electrons, and the ionic compounds they form. Calcium and sulfur which one of the listed elements will form an ion with a 2+ charge?
 Source: youtube.com
Source: youtube.com
Which pair of elements will form an ionic compound. Mg is a metal and f is a nonmetal. Which pair of elements is most likely to form an ionic compound?
 Source: oneclass.com
Source: oneclass.com
Ionic compounds generally form between elements that are metals and elements that are nonmetals. Magnesium and chlorine will form an ionic compound. These types of ionic compounds are composed of monatomic cations and anions.
![Solved] Decide Whether Each Pair Of Elements In The Table Below Will Form An Ionic Compound. If They Will, Write The Empirical Formula And Name Of T… | Course Hero](https://www.coursehero.com/qa/attachment/12778210/ “Solved] Decide Whether Each Pair Of Elements In The Table Below Will Form An Ionic Compound. If They Will, Write The Empirical Formula And Name Of T… | Course Hero”) Source: coursehero.com
Which compound contains ionic bonds? Name of ionic compound empirical formula of lonic compound o sulfur cesium ves ano ? 6.11 which of the following pairs of elements are likely to form an ionic compound?
 Source: brainly.in
Source: brainly.in
Element 1 element #2 forms lonic compound? Which pair of elements is most likely to kind an ionic compound?sodium and aluminumoxygen and chlorine nitrogen and also sulfurmagnesium and also fluorine magnesium and also flourinemetals and nonmetals often tend to type ionic bonds. Metals and nonmetals tend to form ionic bonds.
 Source: numerade.com
Source: numerade.com
The chart below shows monatomic ions formed when an atom loses or gains one or more electrons, and the ionic compounds they form. For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl2). Ionic compounds generally form between elements that are metals and elements that are nonmetals.
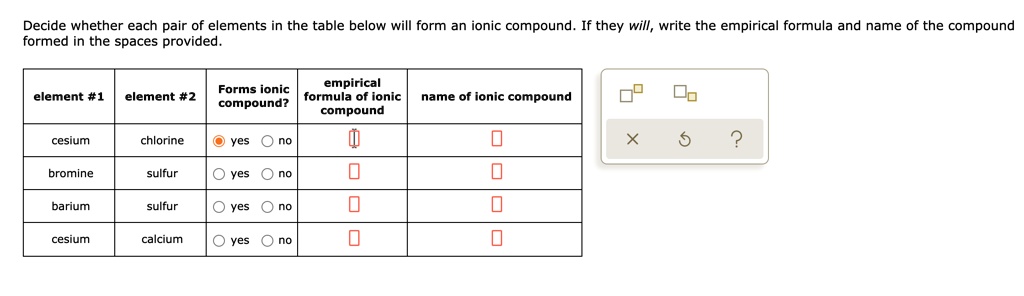 Source: numerade.com
Source: numerade.com
For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl 2 ). K looses one electron to form monoatomic ion; Oxygen forms the oxide to minus signs, so this would form a non metal iron.
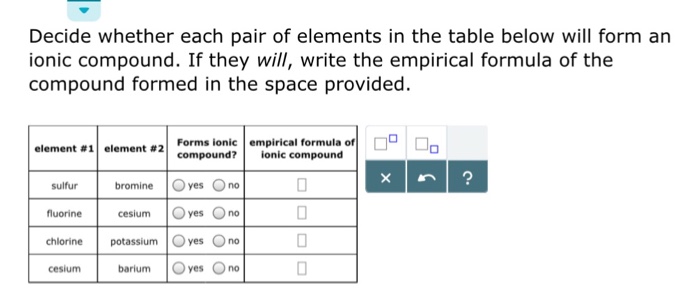
These types of ionic compounds are composed of monatomic cations and anions. For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl 2 ). Which of the following pairs of elements is most likely to form an ionic compound?.

Ionic compounds generally form between elements that are metals and elements that are nonmetals. A) pcl 5 b) crcl 6 c) rbcl d) pbcl 2 e) nacl An ionic bond forms between atoms of a.
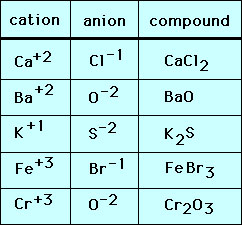 Source: socratic.org
Source: socratic.org
What elements can form an ionic compound? For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl 2 ). For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl 2).
 Source: youtube.com
Source: youtube.com
Ionic compounds generally form between elements that are metals and elements that are nonmetals. Which pair of elements is most likely to form an ionic compound? Nonmetals and nonmetals tend to form covalent bonds.
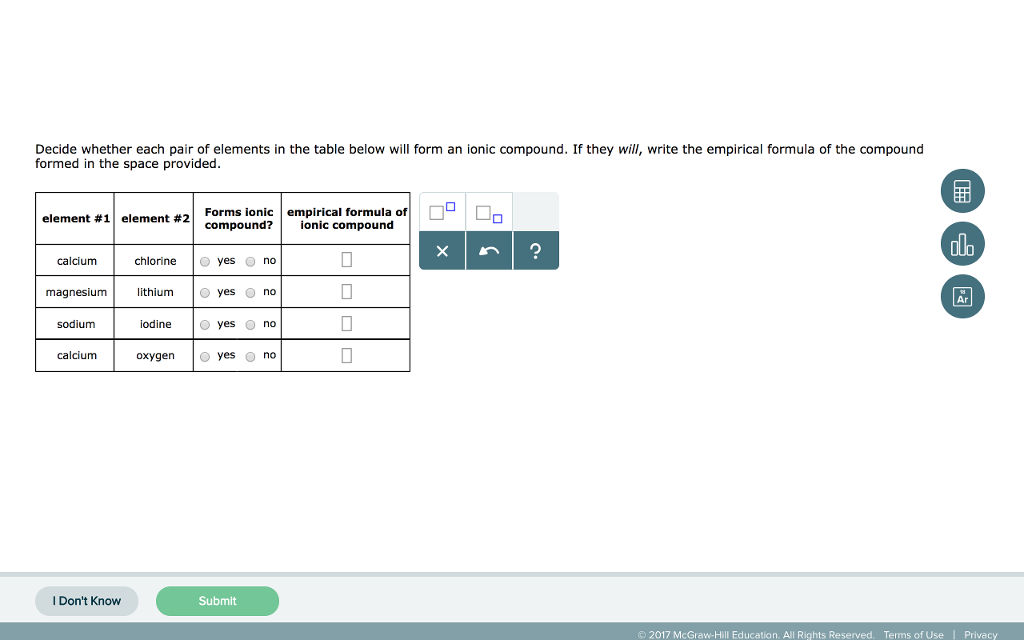 Source: chegg.com
Source: chegg.com
6.11 which of the following pairs of elements are likely to form an ionic compound? For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl 2). A) barium, chlorine b) calcium, sodium c) oxygen, fluorine d) sulfur, carbon e) nitrogen, hydrogen 23)of the choices below, which one is not an ionic compound?
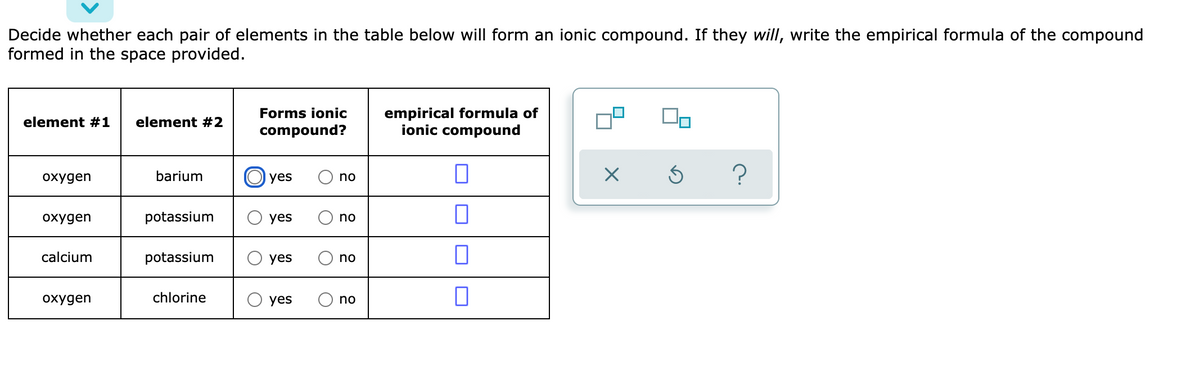 Source: bartleby.com
Source: bartleby.com
For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl 2). These types of ionic compounds are composed of monatomic cations and anions. Let’s imagine what happens when lithium reacts with chlorine to form an ionic compound.
 Source: slideplayer.com
Source: slideplayer.com
K + and i gains one electron to form i −. For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl2). Calcium and sulfur which one of the listed elements will form an ion with a 2+ charge?
 Source: brainly.com
Source: brainly.com
Which pair of elements will form an ionic compound. Which pair of ions will form an ionic compound with a 1 1 ratio? For example, the metal calcium (ca) and the nonmetal chlorine (cl) form the ionic compound calcium chloride (cacl2).
 Source: chegg.com
Source: chegg.com
Sulfur loding yes no 0 0 barium cesium yes no 0 Which one of the following pairs will form an ionic compound with a 1:1 ratio between the cation and anion? Let’s imagine what happens when lithium reacts with chlorine to form an ionic compound.
 Source: brainly.com
Source: brainly.com
In this compound, there are two negative chloride ions for each positive calcium ion. Name of ionic compound empirical formula of lonic compound o sulfur cesium ves ano ? A pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal.
Also Read :





