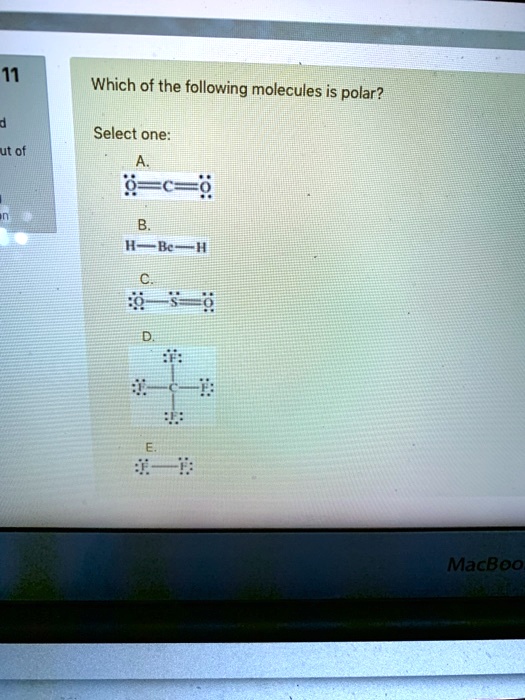
Which of the following molecules is nonpolar? Which of the following is true of the molecule o=c=s?
Which One Of The Following Molecules Is Polar. B.ethylene has one triple bond. When atoms come together in chemical bonding, they share electrons. Such a bond is known as a polar bond. Xef4, brf5, ccl4, xef2, or pbr5?
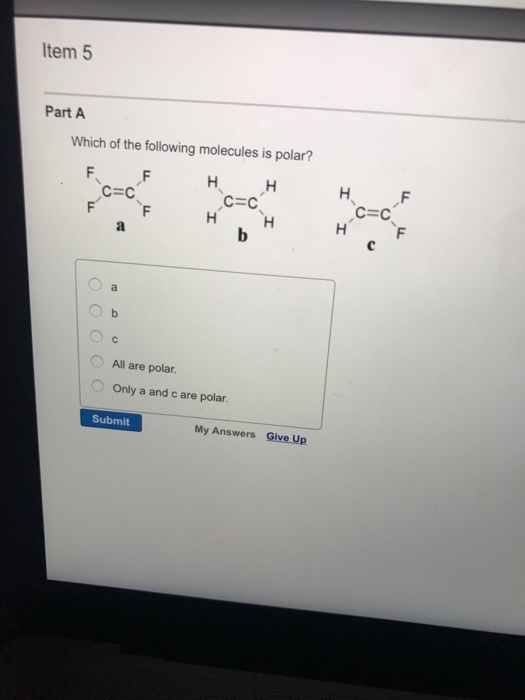
Related Post Solved Which Of The Following Molecules Is Polar? A B C | Chegg.com :
A) o=c=s is nonpolar because it contains only double bonds. Has the highest permanent electric dipole, chcl3, sf6, sncl4, bf3, co2. Other polar compounds interact with polar molecules. The shapes that are symmetrical are polar.
Has two polar bonds, but the polar bonds in the bent molecule results in a net dipole moment making.
The electronegativity gap between the atoms is bigger than 0.4. A) o=c=s is nonpolar because it contains only double bonds. A) becl2 b) br2 c) bf3 d) ibr e) co2 Which of the following is polar molecule? B) o=c=s is nonpolar because it is linear. Ch4 is also not polar as there is no imbalance in the charge of the molecules.
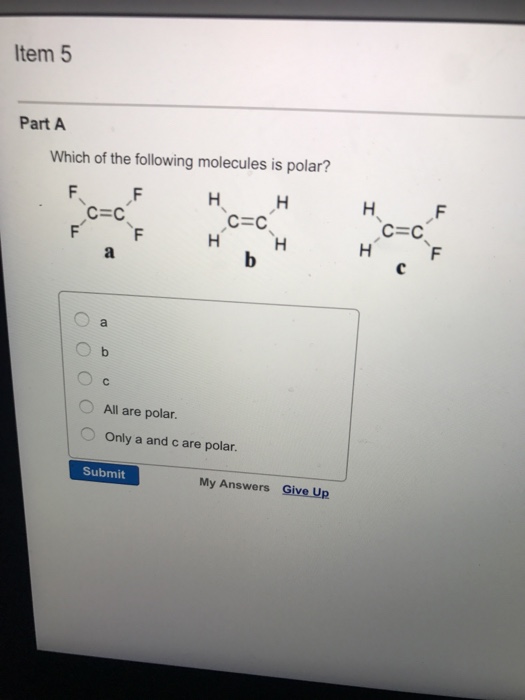
A polar molecule is a molecule or a compound with polar bonds and the molecular geometry that allows for that. Which one of the following molecules is polar? The bonding atoms are the same and hence there is no electronegative difference between them.
 Source: clutchprep.com
Source: clutchprep.com
A) co2 b) bcl3 c) h2o d) n2 e) more than one Learn this topic by watching molecular polarity concept videos. This means that this molecule would no longer be symmetrical and thus this molecule will once again be polar.
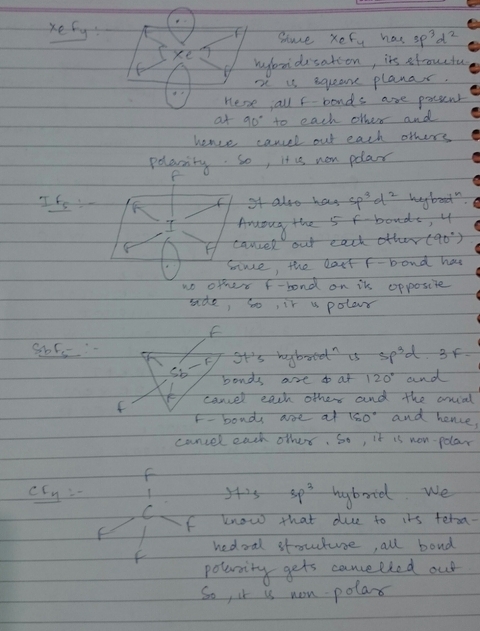 Source: meritnation.com
Source: meritnation.com
Learn this topic by watching molecular polarity concept videos. Oxygen is more electronegative than hydrogen, hence the water molecule is polar in nature. The shapes that are symmetrical are polar.
 Source: clutchprep.com
Source: clutchprep.com
Cbr4 is also not polar because in this case also no imbalance in the molecular charges can be seen. A polar molecule is a molecule or a compound with polar bonds and the molecular geometry that allows for that. A) bh3 b) ccl4 c) so2 d) co2 e) pf5
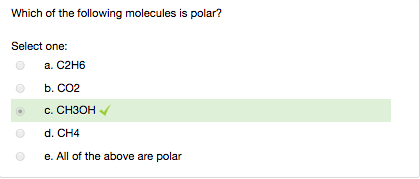 Source: chegg.com
Source: chegg.com
Other polar compounds interact with polar molecules. D) polar bonds, but is a nonpolar molecule. This means that this molecule would no longer be symmetrical and thus this molecule will once again be polar.
 Source: chegg.com
Source: chegg.com
A polar molecule arises when one of the atoms exerts a stronger attractive force on the electrons in the bond. Homework statement which of the following is the most polar molecule? A) bh3 b) ccl4 c) so2 d) co2 e) pf5
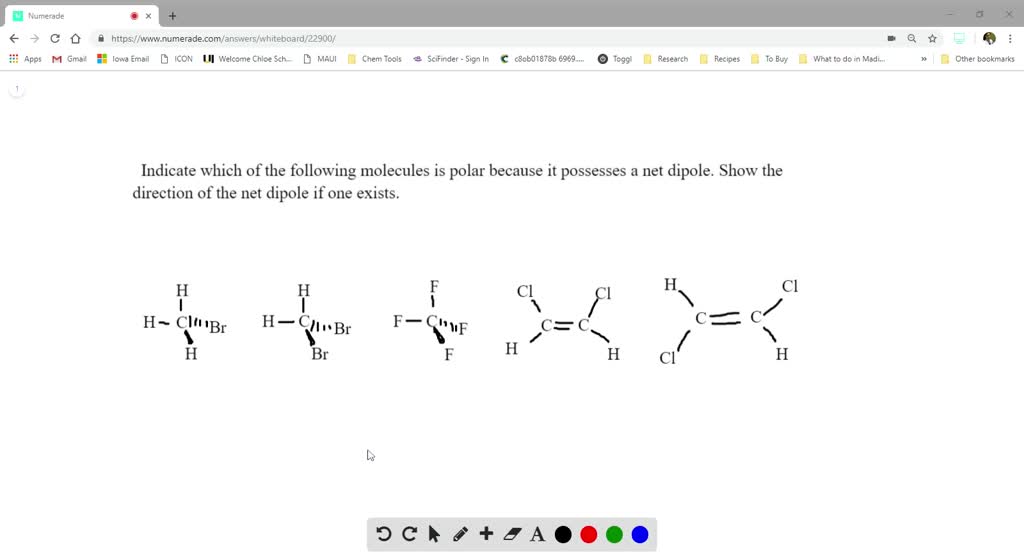 Source: numerade.com
Source: numerade.com
Has the highest permanent electric dipole, chcl3, sf6, sncl4, bf3, co2. Oxygen is more electronegative than hydrogen, hence the water molecule is polar in nature. Of c and but all the four c − c 1 bond dipoles cancel each.
 Source: oneclass.com
Source: oneclass.com
As you can see the presence of this hydrogen means that this molecule is not symmetrical. In c c l 4, there is a large difference between the electronegativity? D.none of these is correct.
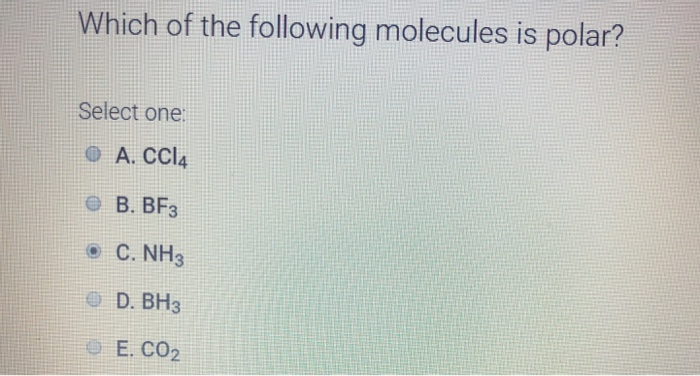 Source: chegg.com
Source: chegg.com
Cbr4 is also not polar because in this case also no imbalance in the molecular charges can be seen. Check answer and solution for above question from ch Is a polar molecule due to large electronegativity difference, so electrons transfer from sodium to chlorine atom completely.
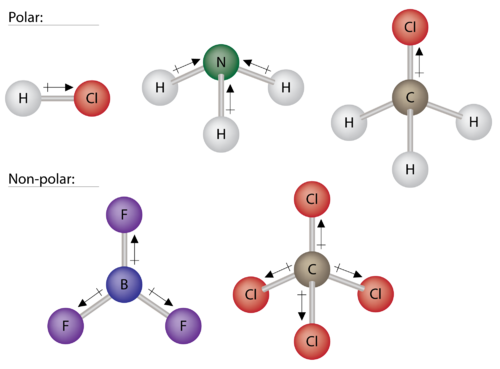 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Is a polar molecule due to large electronegativity difference, so electrons transfer from sodium to chlorine atom completely. Has two polar bonds, but the polar bonds in the bent molecule results in a net dipole moment making. A) becl2 b) br2 c) bf3 d) ibr e) co2

The final molecule we have is chc three. C) polar bonds, and is a polar molecule. The four chlorine atoms are positioned symmetrically at the four corners of a tetrahedron, and a single bond joins each of them to the carbon atom in the center of the molecule.
 Source: toppr.com
Source: toppr.com
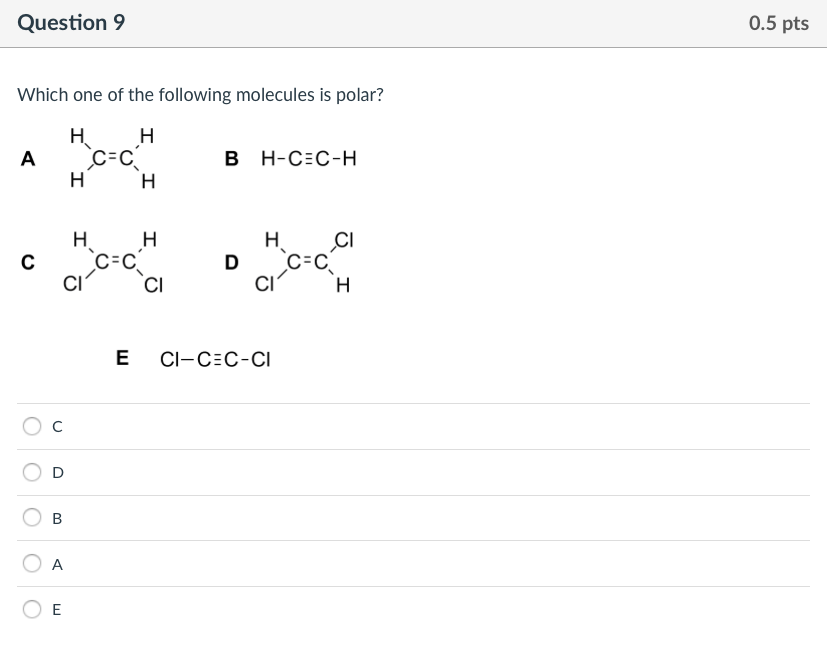
Which one of the following molecules is polar? Check answer and solution for above question from ch A.ethylene has only single bonds.
 Source: toppr.com
Source: toppr.com
B) o=c=s is nonpolar because it is linear. The final molecule we have is chc three. Out of these molecules brf5 is a polar molecule.it has square pyramidal geometry ,four �f� atoms cancel out each other�s effect ,but fifth one has dipole moment ,that.
 Source: brainly.com
Source: brainly.com
A) bh3 b) ccl4 c) so2 d) co2 e) pf5 D.none of these is correct. Which one of the following molecules is polar:
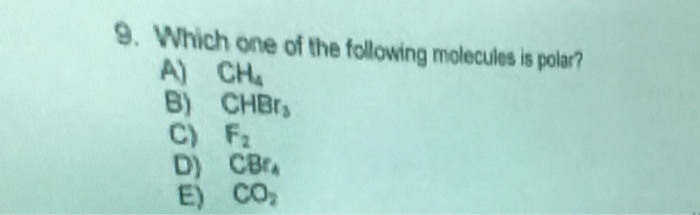
A) o=c=s is nonpolar because it contains only double bonds. This image sums it up: Learn this topic by watching molecular polarity concept videos.
 Source: numerade.com
Source: numerade.com
Has two polar bonds, but the polar bonds in the bent molecule results in a net dipole moment making. A) bh3 b) ccl4 c) so2 d) co2 e) pf5 The melting point, surface tension, boiling point, and vapour pressure of polar bonding are all high.
 Source: geometryofmolecules.com
Source: geometryofmolecules.com
Which one of the following molecules is polar: Which one of the following molecules is polar? Has the highest permanent electric dipole, chcl3, sf6, sncl4, bf3, co2.

A) o=c=s is nonpolar because it contains only double bonds. As you can see the presence of this hydrogen means that this molecule is not symmetrical. To determine whether a molecule is polar or nonpolar draw the lewis diagram then determine the geometry of each molecules.
 Source: clutchprep.com
Source: clutchprep.com
Cc14 which of the following molecules is nonpolar? Which of the following is polar molecule? The symmetrical shapes are not polar unless one of the binding sites is a different atom than then others.
 Source: chegg.com
Source: chegg.com
Learn this topic by watching molecular polarity concept videos. C) polar bonds, and is a polar molecule. 30.ethylene has the formula c 2 h 4.
Also Read :





