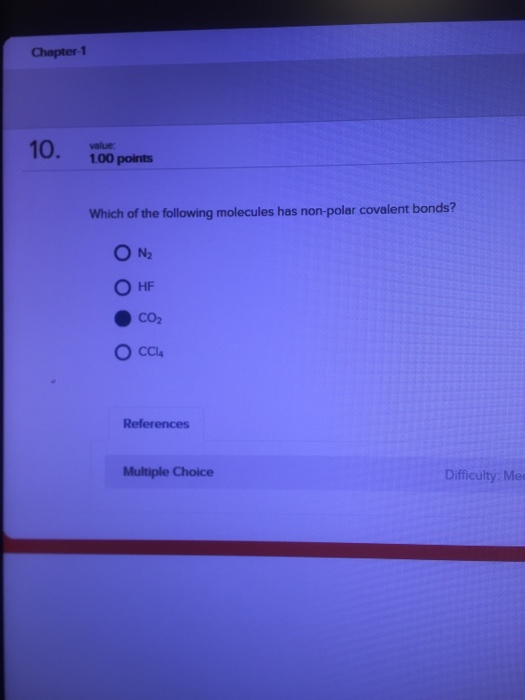
30.ethylene has the formula c 2 h 4. Which one of the following molecules is nonpolar?
Which One Of The Following Molecules Is Nonpolar. P cl5 p c l 5. A) nonpolar bonds, and is a nonpolar molecule. A) all diatomic molecules are polar. Can you please answer this question:
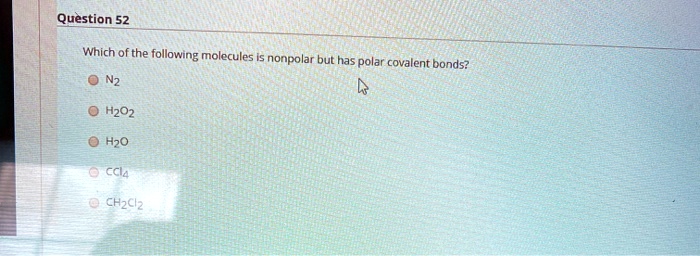 Solved:question 52 Which Of The Following " Molecules Nonpolar But Has Polar Covalent Bonds? H202 H2O Cci4 Chzclz From numerade.com
Solved:question 52 Which Of The Following " Molecules Nonpolar But Has Polar Covalent Bonds? H202 H2O Cci4 Chzclz From numerade.com
Related Post Solved:question 52 Which Of The Following " Molecules Nonpolar But Has Polar Covalent Bonds? H202 H2O Cci4 Chzclz :
Which one of the following molecules is nonpolar? C.ethylene has one double bond. Chemical bonding and molecular structure. As a result, they are nonpolar molecules by nature (examples:
P cl5 p c l 5.
1)a large nonpolar molecule is likely to be. B) all linear triatomic molecules are nonpolar. 29.which one of the following molecules is nonpolar? A) nonpolar bonds, and is a nonpolar molecule. The next molecule that we have is our dime on a fluoride. Predict the molecular geometry and polarity of the so 2 molecule.
 Source: numerade.com
Source: numerade.com
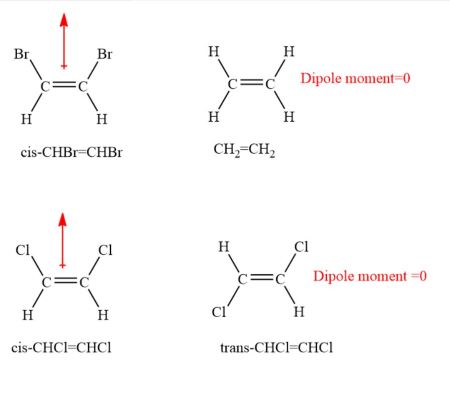
Molecules with less than eight electrons; Explain why one of these molecules is polar and the other is nonpolar? All polar molecules have dipole moments.
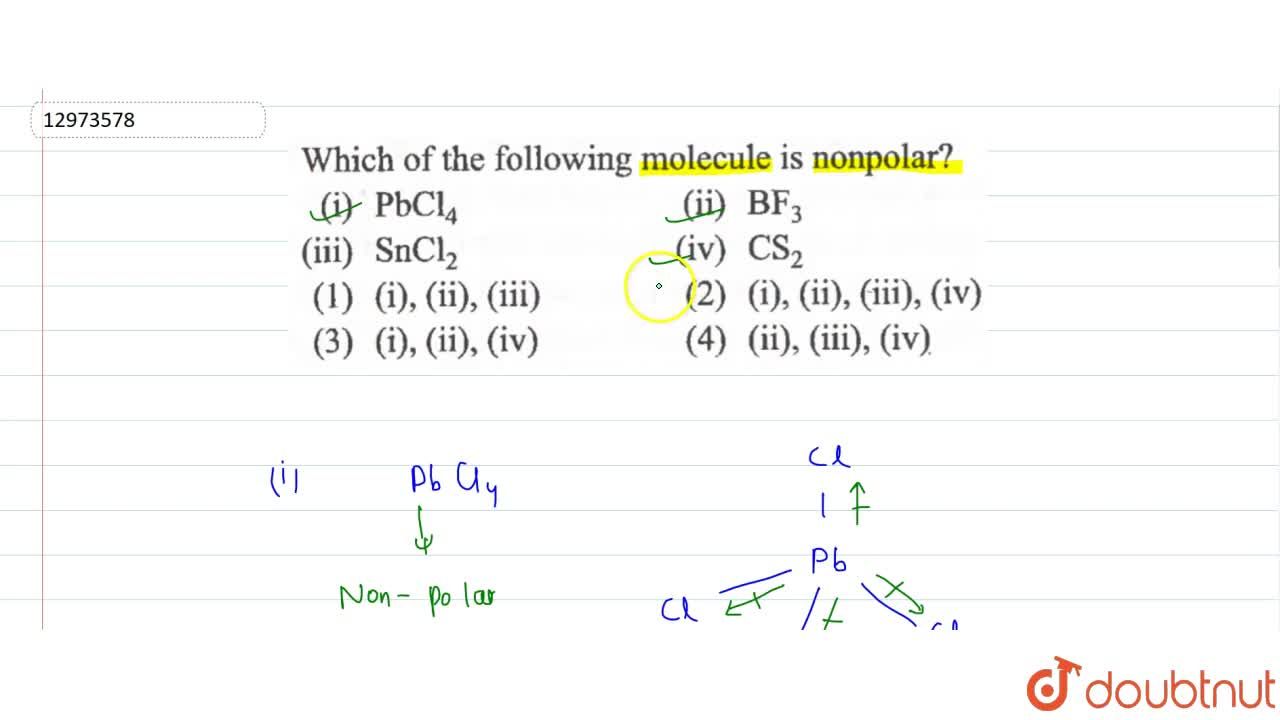 Source: doubtnut.com
Source: doubtnut.com
A) linear, polar d) bent. Of c and but all the four c − c 1 bond dipoles cancel each. Which of the following statements concerning molecular polarity is correct?

The phosphorus pentachloride molecule is nonpolar and contains no lone (unshared) electron pairs on the phosphorus atom. Become a study.com member to unlock this answer! C.ethylene has one double bond.
 Source: study.com
Source: study.com
An example is the bf3. As a result, they are nonpolar molecules by nature (examples: However, because this molecule is symmetrical, all the diaper would cancel each other out.

C) triatomic molecules in which all bonds are polar must be polar. A) nh3 b) of2 c) ch3cl d) h2o e) becl2. Become a study.com member to unlock this answer!
31.what is the name of no 2? Which one of the following molecules/ions is nonpolar? Predict the molecular geometry and polarity of the so 2 molecule.
 Source: chegg.com
Source: chegg.com
All polar molecules have dipole moments. And because there is this known that dipole, it means it means that this molecule is non polar. Which one of the following molecules is nonpolar?
 Source: clutchprep.com
Source: clutchprep.com
Lipids are hydrophobic because the lipid molecules are nonpolar cholesterol, testosterone, progesterone, and the estrogens are nonpolar molecules composed of 4 ring structures. B) nonpolar bonds, but is a polar molecule. It is nonpolar because there is not a significant difference in the electronegativities of its atoms and the.
Which one of the following molecules is nonpolar? A) nonpolar bonds, and is a nonpolar molecule. An example is the bf3.
 Source: studylib.net
Source: studylib.net
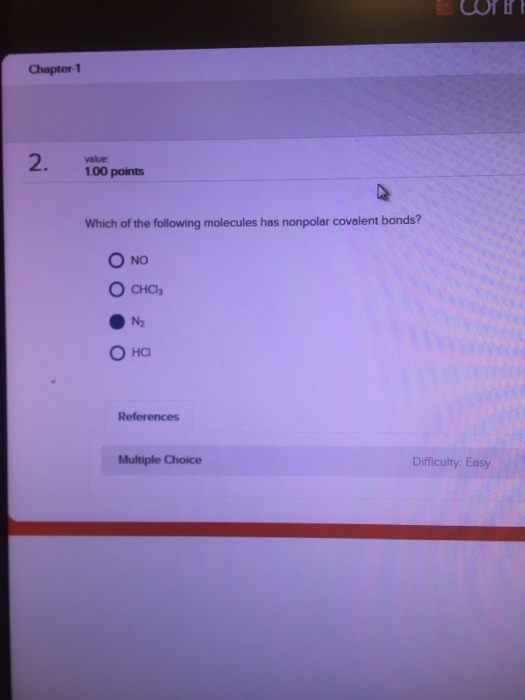
A) all diatomic molecules are polar. Which of the following molecules is nonpolar? Explain why one of these molecules is polar and the other is nonpolar?
 Source: clutchprep.com
Source: clutchprep.com
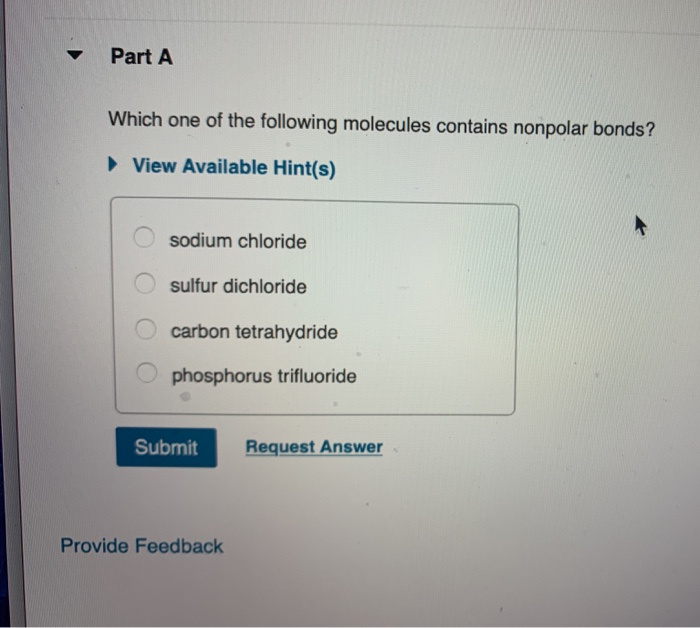
Part a which one of the following molecules contains nonpolar bonds? Which of the following molecules is nonpolar? C) polar bonds, and is a polar molecule.
 Source: numerade.com
Source: numerade.com
Identify the angles between electron groups given the electron geometry. 1)a large nonpolar molecule is likely to be. Be soluble in nonpolar solvents.
 Source: studylib.net
Source: studylib.net
Recall that when a central atom is surrounded by the same element and doesn’t have any lone pairs, the molecule is nonpolar. A) nh b) pci, c) h2o d) o, e carbon dioxide molecule is linear. C) triatomic molecules in which all bonds are polar must be polar.
 Source: clutchprep.com
Source: clutchprep.com
According to v s e p r theory the repulsion between different pair (lone or bond) of electrons obey the order. Which of the following is a correct description of ethylene? Which of the following statements concerning molecular polarity is correct?
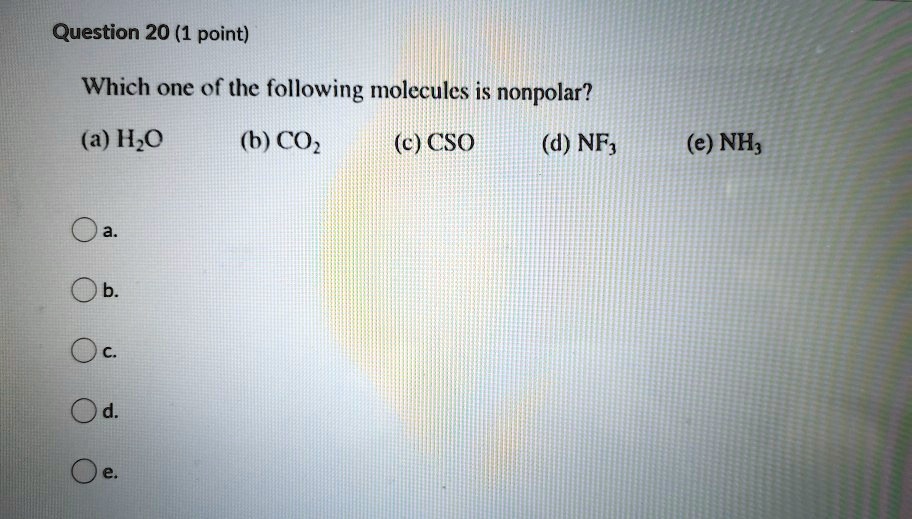 Source: numerade.com
Source: numerade.com
The electronegativities of c and 0 are 39) th 2.5 and 3.5, respectively. To determine which molecule is polar: B.ethylene has one triple bond.
 Source: docplayer.net
Source: docplayer.net
1)a large nonpolar molecule is likely to be. Which one of the following molecules/ions is nonpolar? Which one of the following is a nonpolar molecule with polar covalent bonds?

All polar molecules have dipole moments. A) nh3 b) of2 c) ch3cl d) h2o e) becl2. Which one of the following molecules/ions is nonpolar?
 Source: brainly.com
Source: brainly.com
Which of the following statements is (or are) true? Which of the following is a correct description of ethylene? A) linear, polar d) bent.
 Source: chegg.com
Source: chegg.com
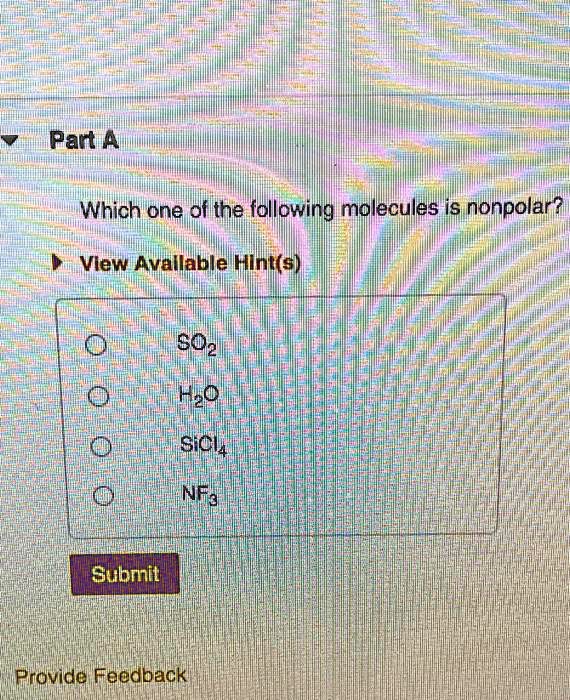
Part a which one of the following molecules contains nonpolar bonds? And therefore has no net. View available hint (s) sodium chloride sulfur dichloride carbon tetrahydride o phosphorus trifluoride submit request answer provide feedback part a which compound describes the strongest attractive forces present in a sample of glucose (c8h1206).
 Source: oneclass.com
Source: oneclass.com
To determine which molecule is polar: The next molecule that we have is our dime on a fluoride. Valence shell electron pair repulsion (vsepr) theory.
Also Read :