Hcooh) acetic acid (chemical formula: Complete the following reaction and identify the brønsted acid.naoh(aq) + hcl(aq) → a.
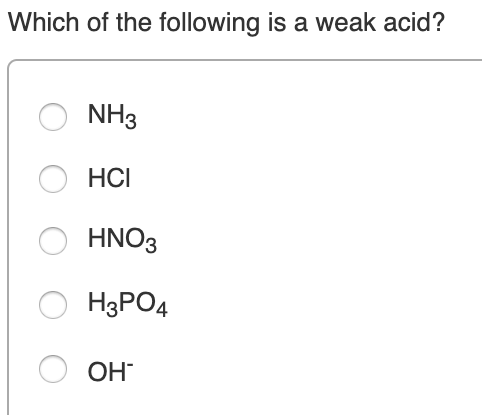
Which One Of The Following Is A Weak Acid. Two acids �a� and �b� were kept in beakers. Has a weak conjugate base e. A strong acid is one that completely ionises in water to form a large amount of hydrogen ions whereas a weak acid only partially ionises in water and thus produces a small amount of hydrogen ions. A weak acid is an acid which on dissociation in an aqueous solution produces low concentration of h+ ions.
 Which Of The Following Is A Weak Acid? A) … | Clutch Prep From clutchprep.com
Which Of The Following Is A Weak Acid? A) … | Clutch Prep From clutchprep.com
Related Post Which Of The Following Is A Weak Acid? A) … | Clutch Prep :
Is slightly dissociated in aqueous solution 27. Consider the following acids and their dissociation constants: Acetic acid (c h 3. Which one of the following is dibasic acid?
Which of the following statements about weak acids is false?
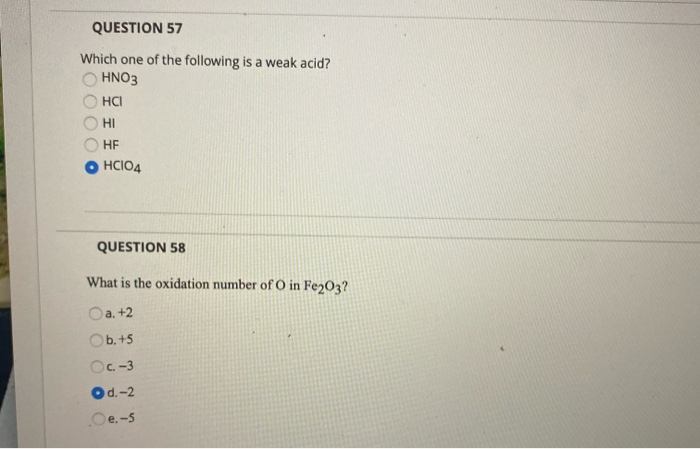
Hcooh) acetic acid (chemical formula: Which of the following is a weak acid? (a) h 3 po 4 (b) h 3 po 3 (c) h 3 bo 3 (d) h 3 aso 4. (a) hno 3 (b) hi (c) hbr (d) hf (e) hclo 3 2. Which one of the following is a weak acid? C 2 h 2 o 4 ) hydrofluoric acid (chemical formula:

Which one of the following is dibasic acid? Iv) give one example of each. C 6 h 5 cooh) oxalic acid (chemical formula:
 Source: thoughtco.com
Source: thoughtco.com
Iv) give one example of each. Iii) what is a strong acid? Which one of the following is dibasic acid?

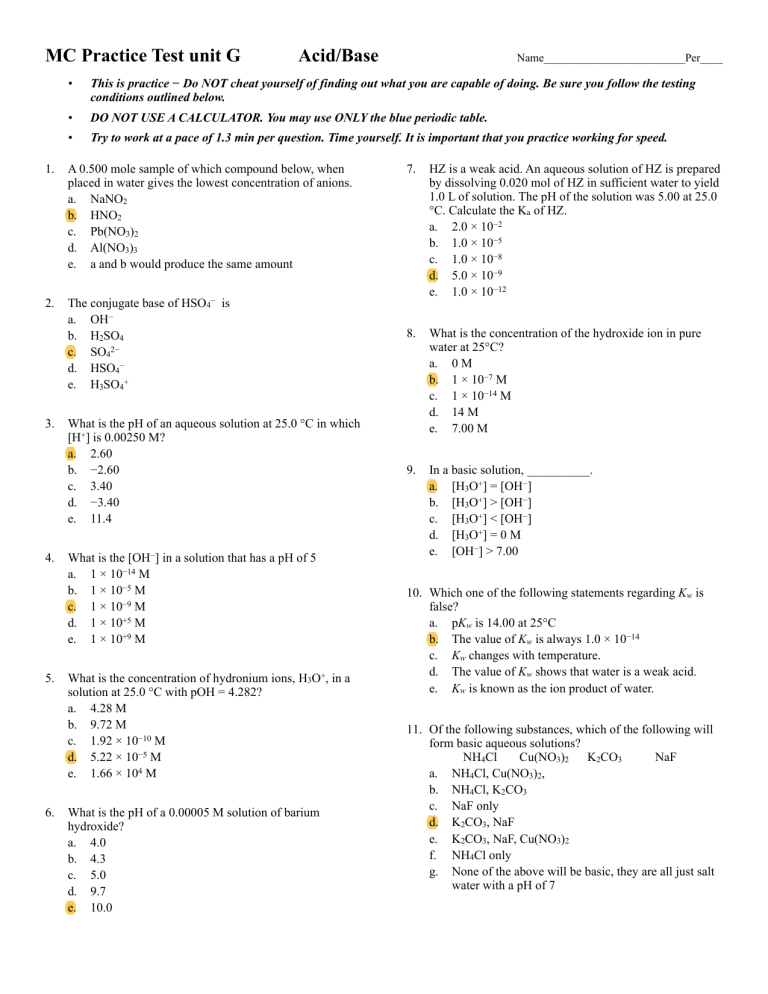
Which of the following acids is a weak acid? Acetic acid (c h 3. Acids capable of yielding mare than one hydronium ion per molecule are called polybasic acids, the dibasic, tribasic etc indicating the number of replaceable hydrogen.

(a) ca(oh) 2 (b) cu(oh) 2 (c) ga(oh) 3 (d) zn(oh) 2 (e) zr(oh) 3. I) of the two acids �a� and �b�, which is weak acid and which is strong acid? At what concentration ratio of sodium acetate to acetic acid would the indicator show a colour half way between those of its acid and conjugate base forms?
 Source: bartleby.com
Source: bartleby.com
Which of the following acids will have the strongest conjugate base? A weak acid is an acid which on dissociation in an aqueous solution produces low concentration of h+ ions. C 2 h 2 o 4 ) hydrofluoric acid (chemical formula:
 Source: pt.slideshare.net
Source: pt.slideshare.net
A strong acid is one that completely ionises in water to form a large amount of hydrogen ions whereas a weak acid only partially ionises in water and thus produces a small amount of hydrogen ions. Which one of the following is likely to be the most soluble base? Answer true or false for each of the following:

Ii) what is a weak acid? Acid �a� undergoes partial dissociation in water, whereas acid �b� undergoes complete dissociation in water. C 2 h 2 o 4 ) hydrofluoric acid (chemical formula:

Which one of the following is a weak acid? At what concentration ratio of sodium acetate to acetic acid would the indicator show a colour half way between those of its acid and conjugate base forms? H c l , h 2 s o 4 and h n o 3 are strong acids.
 Source: chegg.com
Source: chegg.com
I) of the two acids �a� and �b�, which is weak acid and which is strong acid? (a) one mole of any acid will ionize completely in aqueous solution to produce one mole of h + ions. Which one of the following is a weak acid?

Two acids �a� and �b� were kept in beakers. Which one of the following is a weak acid? Iv) give one example of each.
 Source: clutchprep.com
Source: clutchprep.com
C 6 h 5 cooh) oxalic acid (chemical formula: Hcooh) acetic acid (chemical formula: The ka values for weak acids are numbers that are greater than 1 c.
 Source: numerade.com
Source: numerade.com
Which one of the following is a weak acid? C ooh), formic acid (h c ooh), carbonic acid (h 2. Which one of the following is a weak acid?
 Source: chegg.com
Source: chegg.com
Iii) what is a strong acid? Which one of the following is an example of a weak acid? Is completely dissociated in aqueous solution b.
 Source: chegg.com
Source: chegg.com
Has a weak conjugate base e. Acetic acid (c h 3. H c l , h 2 s o 4 and h n o 3 are strong acids.
 Source: chegg.com
Source: chegg.com
Click here👆to get an answer to your question ️ which compound is a weak acid? Hcooh) acetic acid (chemical formula: Which one of the following is an example of a weak acid?
 Source: studylib.net
Source: studylib.net
Is completely dissociated in aqueous solution b. Iii) what is a strong acid? Which of the following acids is a weak acid?
 Source: numerade.com
Source: numerade.com
(a) h 2 so 4 (b) hclo 3 (c) hf (d) hcl (e) hno 3. But carbonic acid h2co3, dissociate only partially. Is completely dissociated in aqueous solution b.
 Source: clutchprep.com
Source: clutchprep.com
Has a small value of k a c. Which one of the following is an example of a weak acid? I) of the two acids �a� and �b�, which is weak acid and which is strong acid?

(a) ca(oh) 2 (b) cu(oh) 2 (c) ga(oh) 3 (d) zn(oh) 2 (e) zr(oh) 3. Ii) what is a weak acid? So carbonic acid is the weakest among the above acids.

Acids capable of yielding mare than one hydronium ion per molecule are called polybasic acids, the dibasic, tribasic etc indicating the number of replaceable hydrogen. At what concentration ratio of sodium acetate to acetic acid would the indicator show a colour half way between those of its acid and conjugate base forms? Cooh), formic acid (hcooh), carbonic acid (h 2.
Also Read :