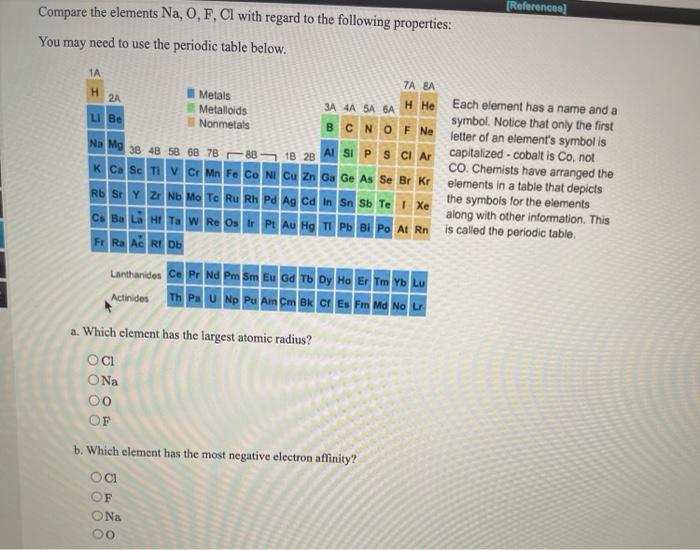
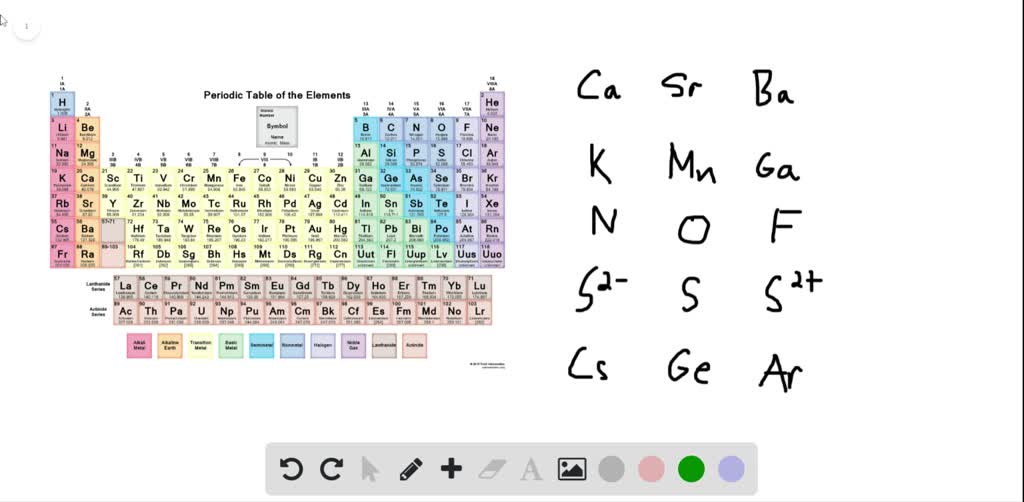
Which one of the following has the smallest radius?a. 0.6 mol of $c_{2} h_{4}$ or 0.6 mol of $f_{2}$ (e) total ions:
Which One Of The Following Has The Smallest Radius. Which of the following correctly lists the five atoms in order of the five atoms in order of increasing size (smallest to largest?) Which of the following is not true about alkali metals? Br is the atom with the smallest. Which of the following atoms has the smallest radius?
Related Post Solved A. In The Following Set, Which Atom Has The Smallest | Chegg.com :
In general, the shape with the smallest radius has the smallest diameter. Based on this you could say: Is f larger than f? See answer (1) best answer.
Heliumhelium has the smallest atomic radius.
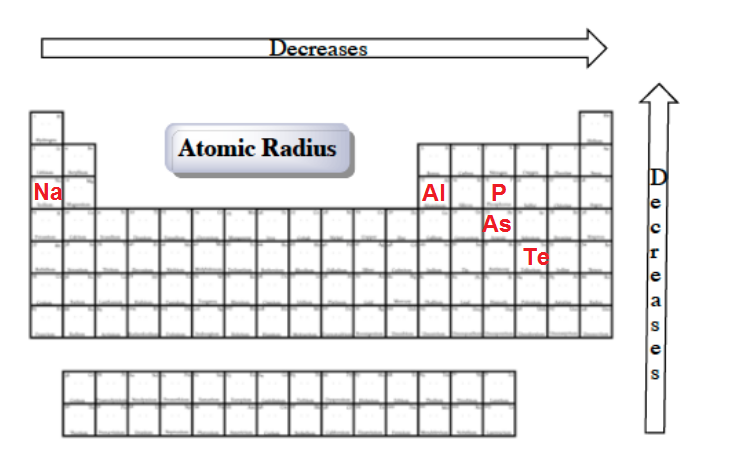
Know course division, evaluation and. Atomic radius decreases as you move across a period from left to right and decreases as you move up a group from bottom to top. Out of k +, sr 2+ and ar, k + has smaller size because it has greater nuclear charge. Where would you find the smallest atom in the periodic table of elements? A) br b) as c) ca d) k. D) they are very reactive elements.

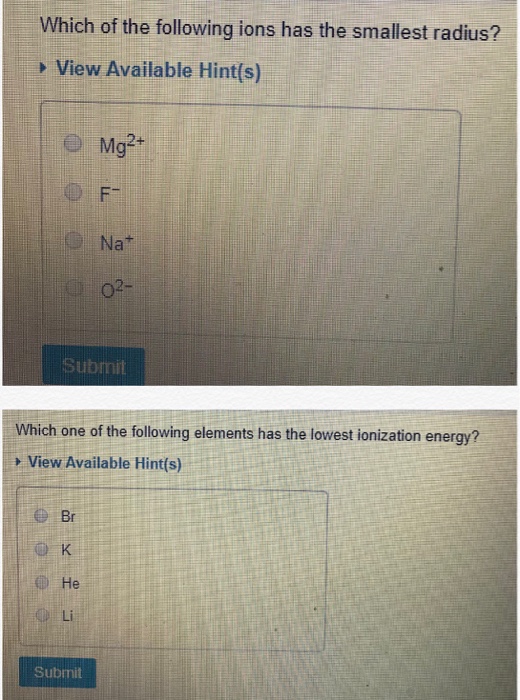
So therefore, elements with a positive charge, will have a smaller ionic radius. Which atom has the highest first ionization energy? This is due to trends in the periodic table, and the effective nuclear charge that holds the valence electrons close to the nucleus.
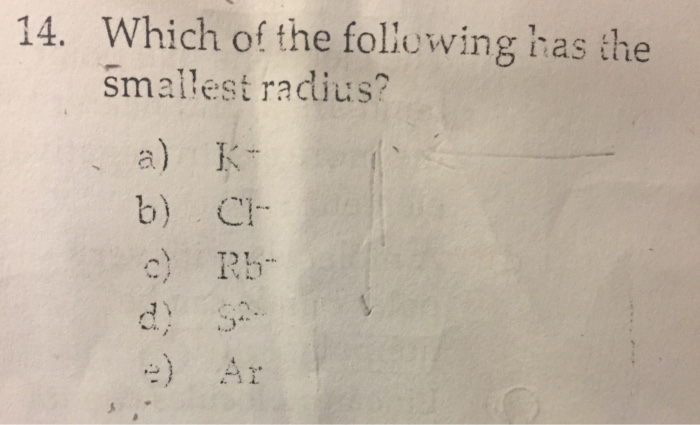
Ii) in k + and ar the outer most shell is third where as in sr 2+ it is fourth. Hpbose class 10 and 12 exam to be held in 2 terms. Element z is larger than element x.
 Source: clutchprep.com
Source: clutchprep.com
C) element z and x are probably in the same group. Which has the smallest atomic radius: Which of the following elements has the largest atomic radius?
 Source: chegg.com
Source: chegg.com
The neet ss is scheduled to be held on 10th january 2022. This is due to trends in the periodic table, and the effective nuclear charge that holds the valence electrons close to the nucleus. See the answer see the answer done loading.
 Source: slideplayer.com
Source: slideplayer.com
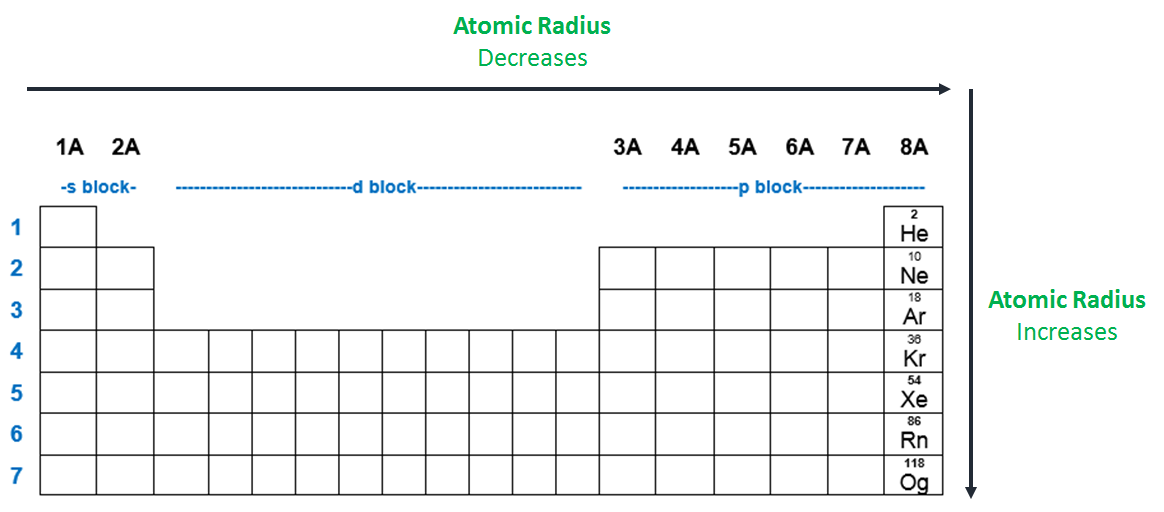
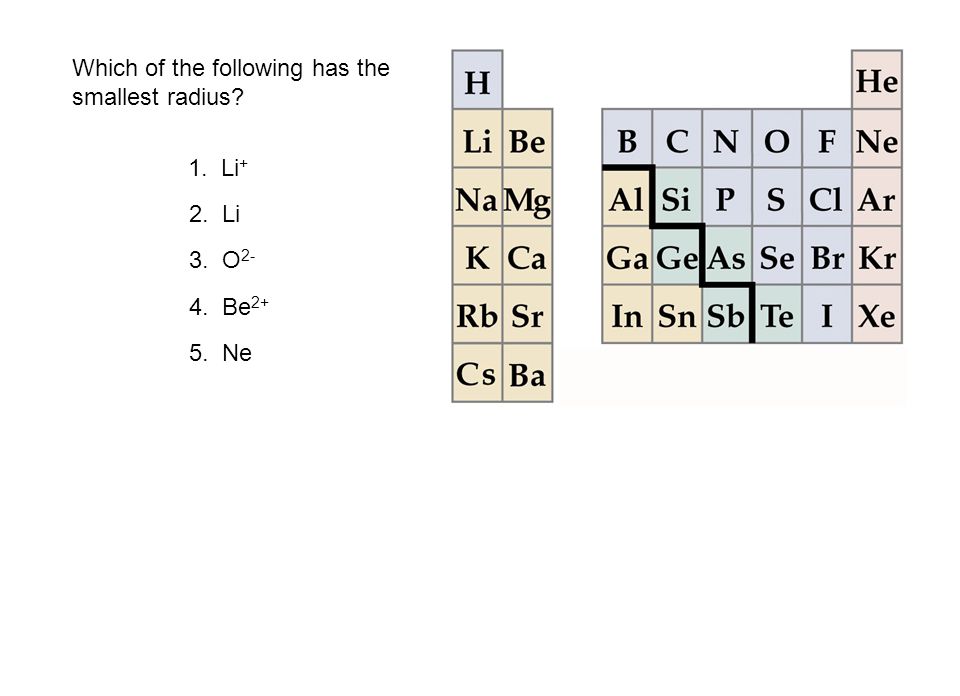
Of the following which gives the current order for the atomic radius for mg, na, p, si, and ar? See the answer see the answer done loading. As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period.
 Source: chegg.com
Source: chegg.com
C) element z and x are probably in the same group. Element x is further to the bottom of the periodic table. We review their content and.
 Source: chegg.com
Source: chegg.com
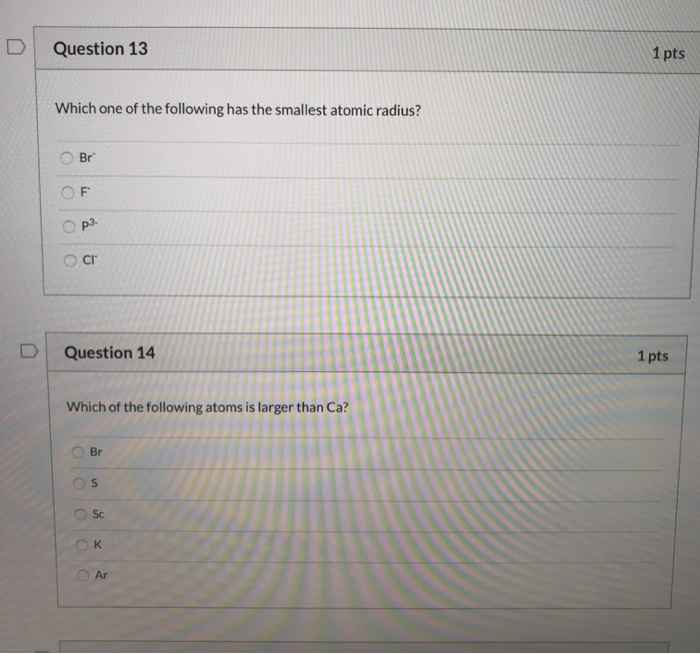
Which of the following atoms has the smallest radius? B) they all readily form ions with a +1 charge. This is due to trends in the periodic table, and the effective nuclear charge that holds the valence electrons close to the nucleus.
 Source: youtube.com
Source: youtube.com
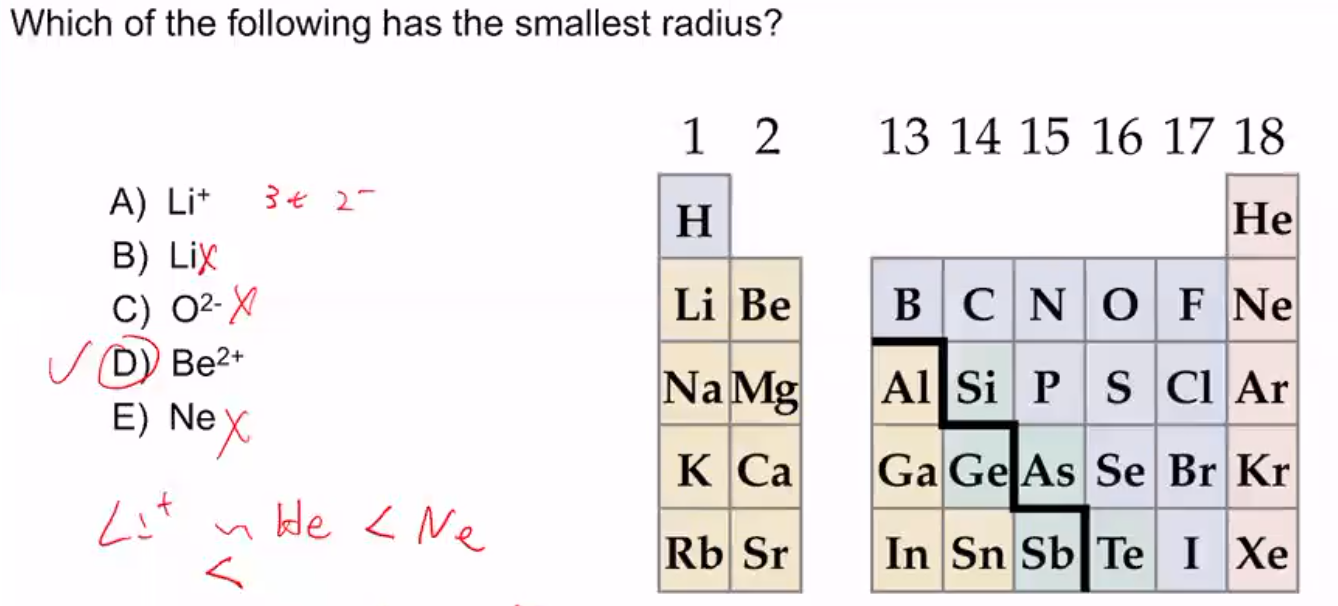
Which one has the smallest radius? C) element z and x are probably in the same group. A microphone at the other end.
 Source: transtutors.com
Source: transtutors.com
C) element z and x are probably in the same group. Which would have a larger atomic radius? Element z is larger than element x.
 Source: chegg.com
Source: chegg.com
Which one of the following has the smallest radius?a. Out of k +, sr 2+ and ar, k + has smaller size because it has greater nuclear charge. Based on this you could say:
 Source: clutchprep.com
Source: clutchprep.com
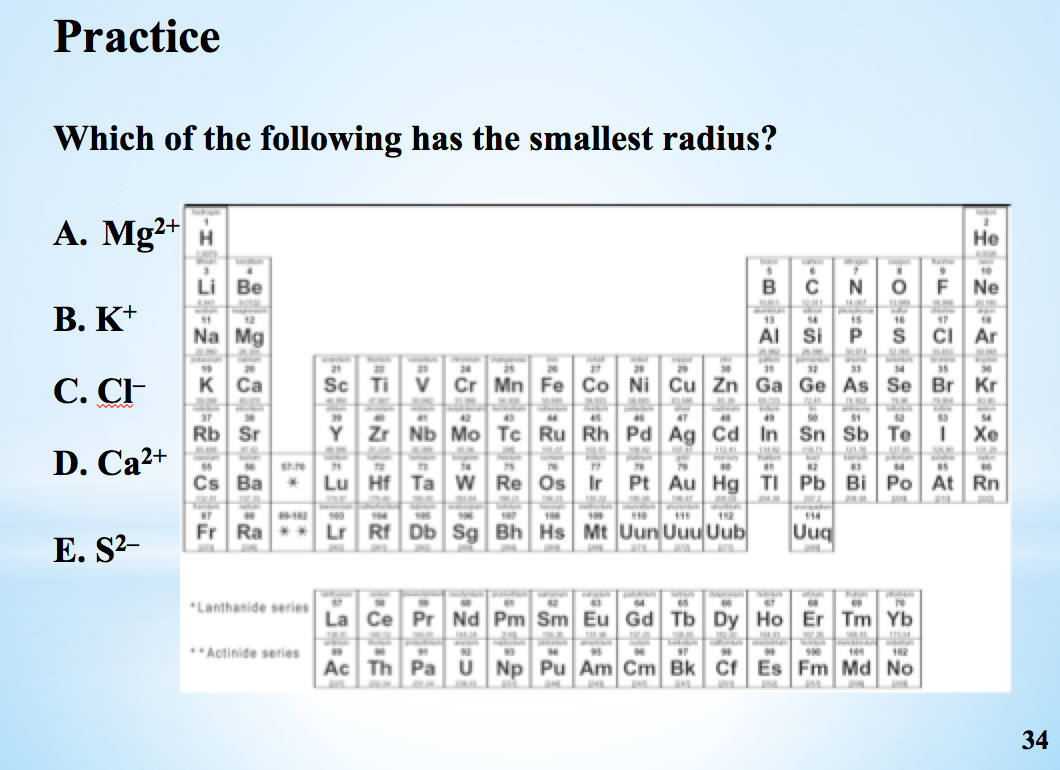
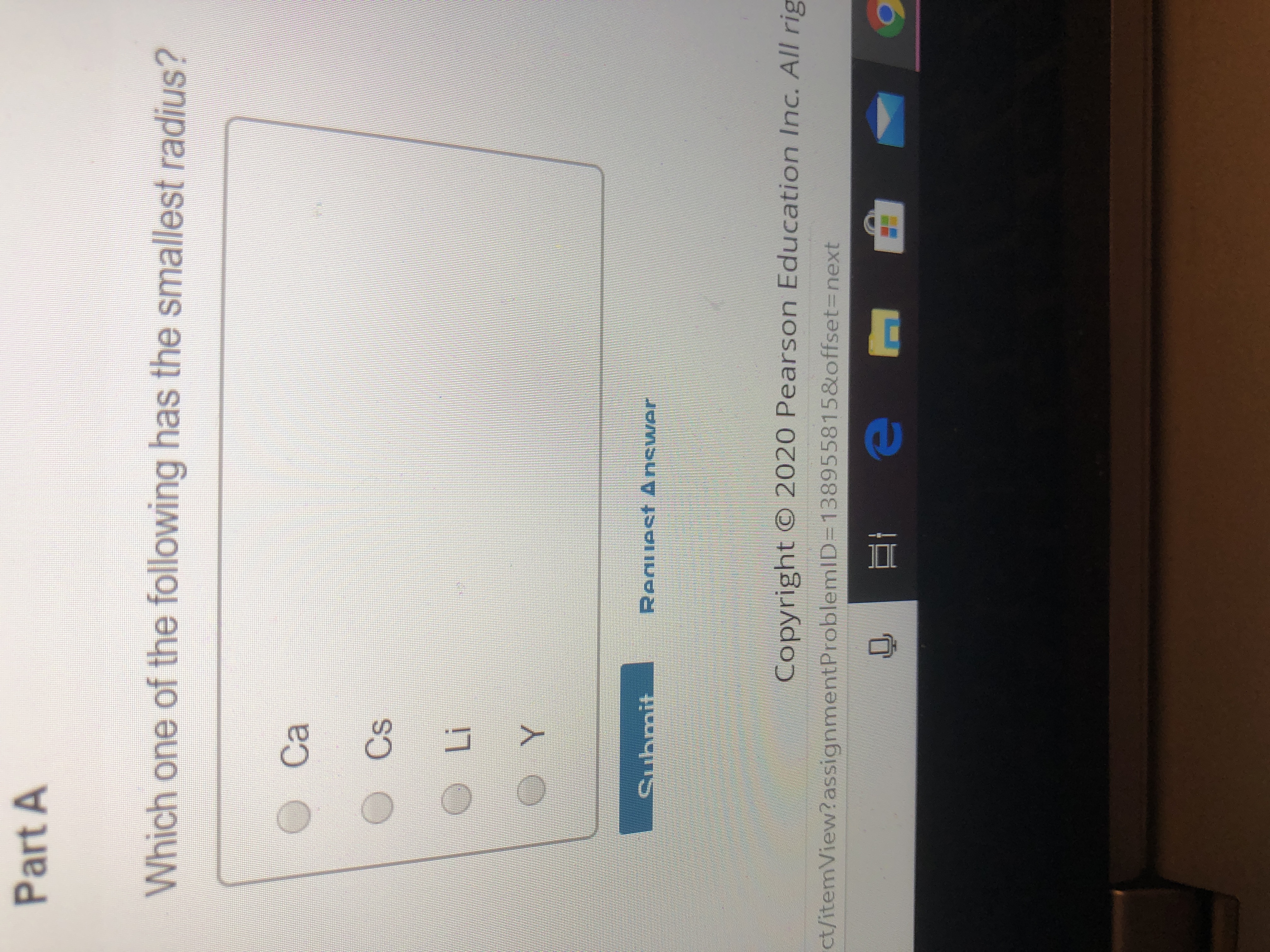
D) they are very reactive elements. Of the following elements, which has the largest first ionization energy? Which one of the following has the smallest radius?

A) na b) al c) n d) f. Experts are tested by chegg as specialists in their subject area. Thus, helium is the smallest element, and francium is the largest.
 Source: youtube.com
Source: youtube.com
A) rb b) si c) s d) o. A) na b) al c) n d) f. Ii) in k + and ar the outer most shell is third where as in sr 2+ it is fourth.

A) na b) al c) n d) f. Which noble gas has the highest first ionization energy? Which of the following atoms has the smallest radius?
 Source: numerade.com
Source: numerade.com
The more positive the charge of an ion is, the smaller the ion is. We review their content and. Element x is further to the bottom of the periodic table.

Which atom has the highest first ionization energy? In isoelectronic species ion with highest positive charge will be smallest in size and ion with highest negative charge will be the highest in size. Nbems has announced the revised schedule of neet ss exam 2021 date.
Experts are tested by chegg as specialists in their subject area. In each pair, choose the larger of the indicated quantities or state that the samples are equal: Elements z and x are compared.
 Source: clutchprep.com
Source: clutchprep.com
A) na b) al c) n d) f. A) they are low density solids at room temperature. Which atom has the lowest first ionization energy?
 Source: bartleby.com
Source: bartleby.com
We review their content and. Hence, c l has the smallest radius. See the answer see the answer done loading.
 Source: clutchprep.com
Source: clutchprep.com
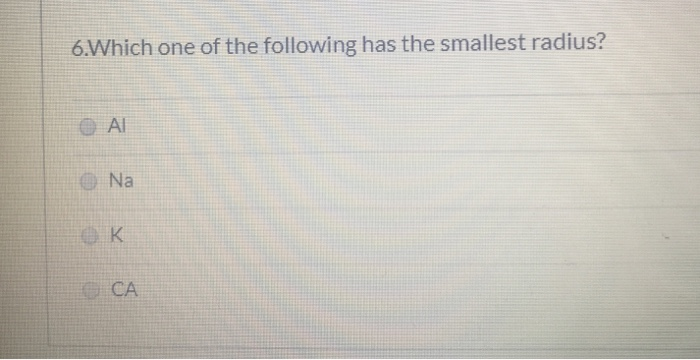
Ii) in k + and ar the outer most shell is third where as in sr 2+ it is fourth. Which of the following atoms has the smallest radius? Which one of the following has the smallest radius?
Also Read :





