The ph is stable because the equilibrium mixture of the ion and the acid or base molecule provides a reservoir of proton acceptors and proton donors that can react with other acids or bases without changing the equilibrium of the hydrogen ion concentration. Which of the following is an arrhenius base?
Which Of These Is An Arrhenius Base. These species can do so either by increasing the concentration of hydronium ions or hydroxide ions in solution. Acid is a compound that dissolves in water releasing h + ions. • an arrhenius acid increases the concentration of h+ ion when dissolved. Learn this topic by watching arrhenius acid and base concept videos.
 Classify Each Of These Compounds As An Arr… | Clutch Prep From clutchprep.com
Classify Each Of These Compounds As An Arr… | Clutch Prep From clutchprep.com
Related Post Classify Each Of These Compounds As An Arr… | Clutch Prep :
The ph is stable because the equilibrium mixture of the ion and the acid or base molecule provides a reservoir of proton acceptors and proton donors that can react with other acids or bases without changing the equilibrium of the hydrogen ion concentration. Kalit gautam · 1 · apr 22 2018. In the solid or pure state, these exist as covalent compounds as the hydrogen ions are generated only when these are mixed with water as per arrhenius acid base theory. 4) which of the following species is amphoteric?
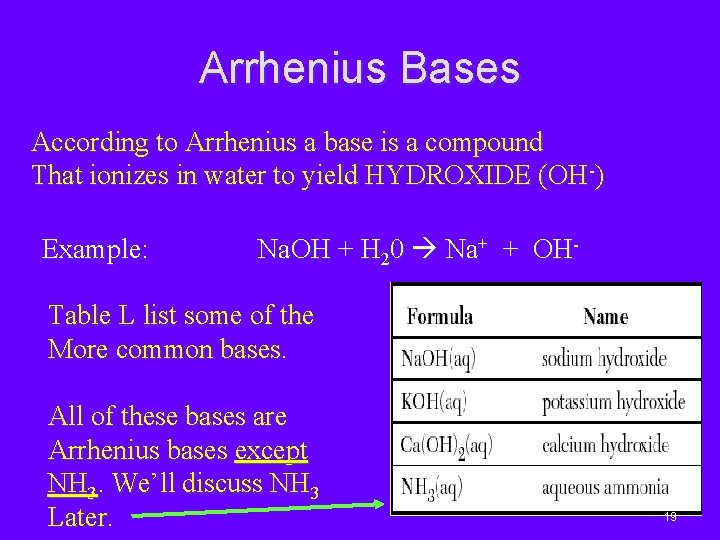
According to him, a substance that gives the proton or a hydrogen ion in their water solution is called an acid.
Arrhenius base is a substance that. Which of the following reactions illustrates amphoterism? Sodium hydroxide, when dissolved in an aqueous. Choose the appropriate option which is true for arrhenius acid. Which of the following is an arrhenius base? Hci(aq) incorrect there is another arrhenius acid.
 Source: slidetodoc.com
Source: slidetodoc.com
Which of these is not an acid, despite being a hydrogen. More than one of these compounds is an arrhenius base. Arrhenius base = koh, lioh,.
 Source: intechopen.com
Source: intechopen.com
• an arrhenius acid increases the concentration of h+ ion when dissolved. To recognize the arrhenius base look for a molecule ending in oh, but not following chx which refers to an alcohol. Arrhenius base is a substance that.
 Source: intechopen.com
Source: intechopen.com
Learn this topic by watching arrhenius acid and base concept videos. Choose the appropriate option which is true for arrhenius acid. Classify each of these compounds as an arrhenius acid, an arrhenius base, or neither.hclo3 kclnaoh hg(oh) 2h3po4 al(oh) 3hcl hbrzn(oh)2 c 3h8.
 Source: flexbooks.ck12.org
Source: flexbooks.ck12.org
Classify each of these compounds as an arrhenius acid, an arrhenius base, or neither. • an arrhenius acid increases the concentration of h+ ion when dissolved. Which of the following is an arrhenius base?
 Source: chegg.com
Source: chegg.com
- which of the following species is amphoteric? According to him, a substance that gives the proton or a hydrogen ion in their water solution is called an acid. The example for arrhenius base is highly soluble sodium hydroxide compound in water, which dissociates to give sodium ion and hydroxide ion.

According to him, a substance that gives the proton or a hydrogen ion in their water solution is called an acid. Kalit gautam · 1 · apr 22 2018. Clear concepts about acids and bases and various theories may help the students to comprehend the chapter well and answer all the questions correctly in neet papers.

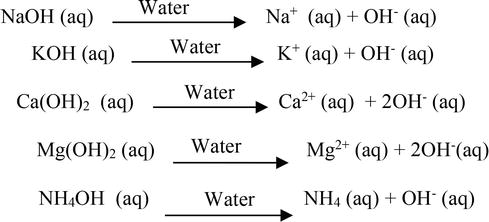
Arrhenius base is a substance that. So arrhenius acid = hcl , hno 3, hbr and hclo. N aoh (aq) → n a+ + oh −.
 Source: slidetodoc.com
Source: slidetodoc.com
We’re being asked to determine which of the following given compounds is an arrhenius base. N aoh (aq) → n a+ + oh −. Classify each of these compounds as an arrhenius acid, an arrhenius base, or neither.hclhbrhcloca(oh)2ch4kohzn(oh)2h3po4agohkcl.
 Source: clutchprep.com
Source: clutchprep.com
A) ch_3 co_2 h b) naoh c) ch_3 oh d) licl e) more than one of these compounds is an arrhenius base. Which of the following reactions illustrates amphoterism? We’re being asked to determine which of the following given compounds is an arrhenius base.

The buffer consists of a mixture of a weak acid or weak base and one of its salts. Which of the following reactions illustrates amphoterism? A) ch3co2h b) koh c) ch3oh d) licl e) more than one of these compounds is an arrhenius base.
 Source: slideplayer.com
Source: slideplayer.com
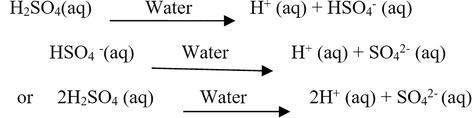
It is possible for an acid to be of more than one type be sure to consider any complex ions that the species may form with water arrhenius acids: So arrhenius acid = hcl , hno 3, hbr and hclo. Recall that according to the arrhenius definition:
 Source: study.com
Source: study.com
Classify each of these compounds as an arrhenius acid, an arrhenius base, or neither.hclo3 kclnaoh hg(oh) 2h3po4 al(oh) 3hcl hbrzn(oh)2 c 3h8. Clear concepts about acids and bases and various theories may help the students to comprehend the chapter well and answer all the questions correctly in neet papers. Because the hydroxide ions are released in the aqueous solution, the hydroxide concentration increases;
 Source: chegg.com
Source: chegg.com
A) ch4 b) hcn c) nh3 d) cl2 e) none of the above are lewis bases. Which of the following reactions illustrates amphoterism? • an arrhenius acid increases the concentration of h+ ion when dissolved.
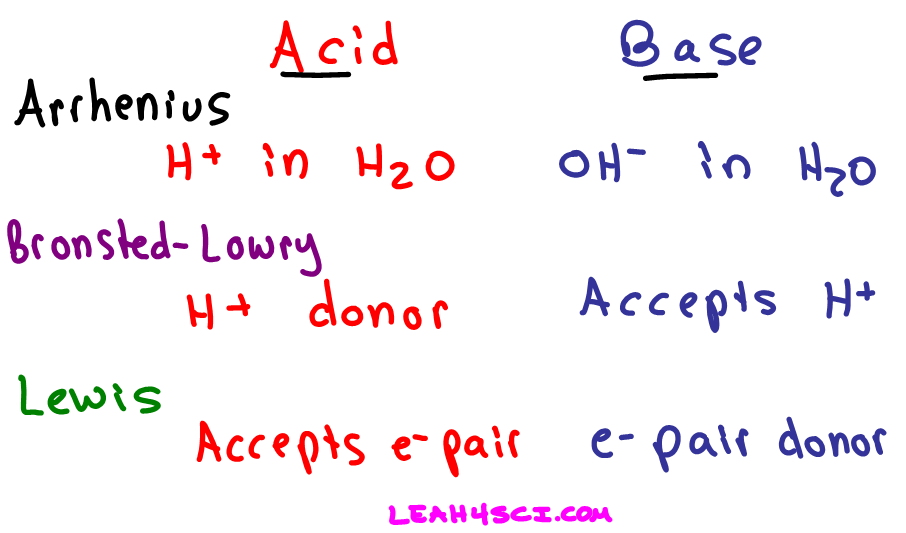 Source: leah4sci.com
Source: leah4sci.com
A base is defined as a compound that dissociates in an aqueous solution liberating oh− (hydroxide ions). It is possible for an acid to be of more than one type be sure to consider any complex ions that the species may form with water arrhenius acids: H 3 po 4 al (oh) 3.

A) ch3co2h b) koh c) ch3oh d) licl e) more than one of these compounds is an arrhenius base. Ch3co2h koh ch3oh nabr more than one of these compounds is an arrhenius base. Clear concepts about acids and bases and various theories may help the students to comprehend the chapter well and answer all the questions correctly in neet papers.
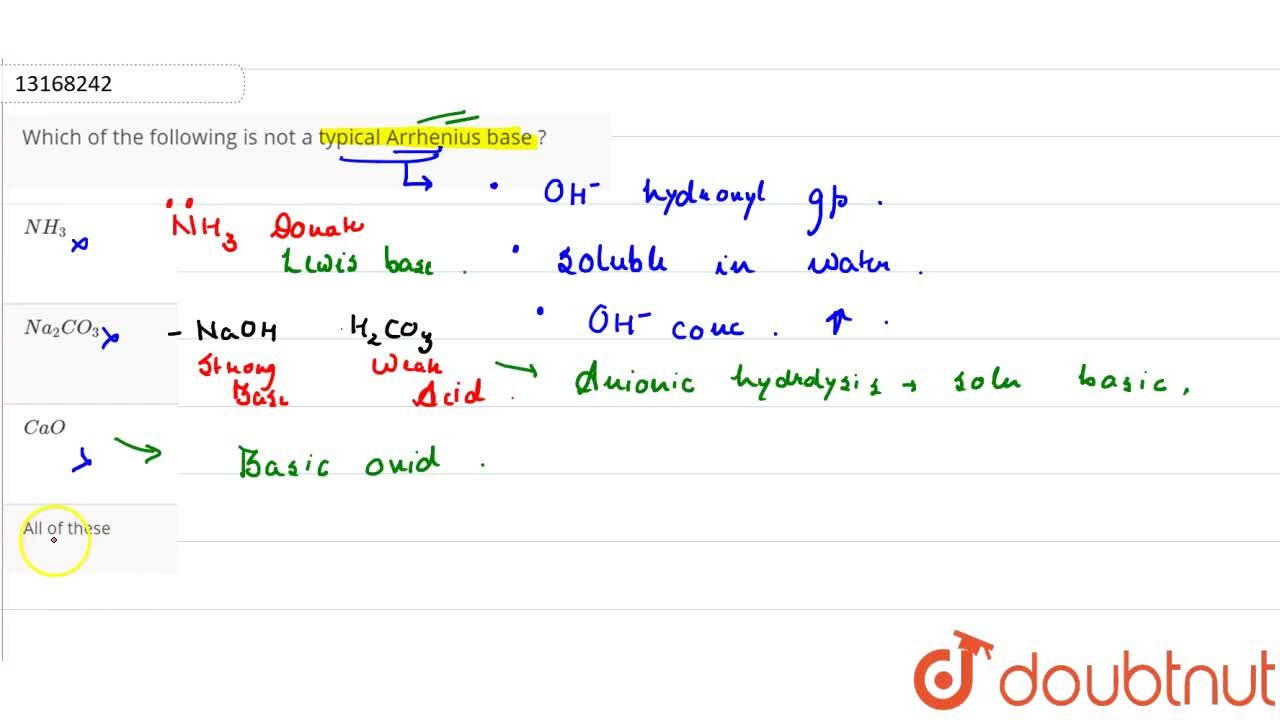 Source: doubtnut.com
Source: doubtnut.com
Sodium hydroxide, when dissolved in an aqueous. Arrhenius base = koh, lioh,. These species can do so either by increasing the concentration of hydronium ions or hydroxide ions in solution.
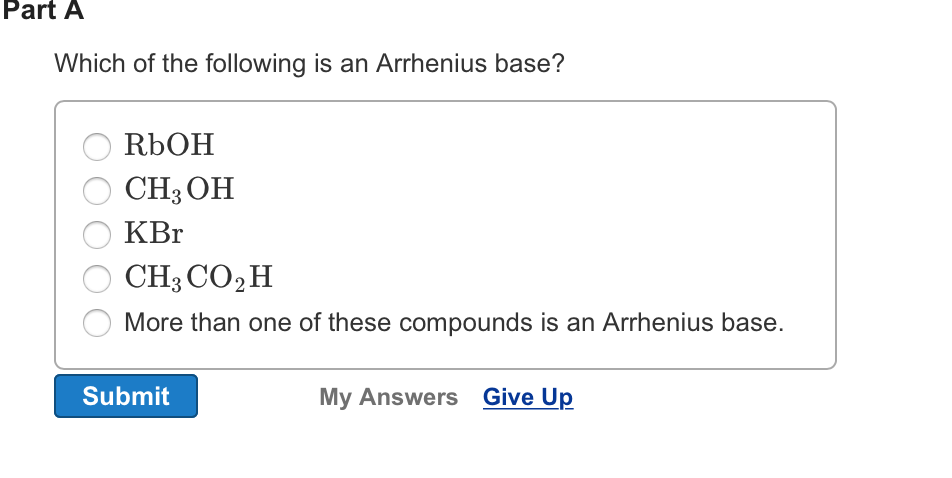 Source: chegg.com
Source: chegg.com
We’re being asked to determine which of the following given compounds is an arrhenius base. We’re being asked to determine which of the following given compounds is an arrhenius base. Mcqs on acids and bases.
 Source: slideplayer.com
Source: slideplayer.com
Mcqs on acids and bases. Classify each of these compounds as an arrhenius acid, an arrhenius base, or neither.hclo3 kclnaoh hg(oh) 2h3po4 al(oh) 3hcl hbrzn(oh)2 c 3h8. Arrhenius base = koh, lioh,.
 Source: clutchprep.com
Source: clutchprep.com
Classify each of these compounds as an arrhenius acid, an arrhenius base, or neither.hclo3 kclnaoh hg(oh) 2h3po4 al(oh) 3hcl hbrzn(oh)2 c 3h8. This is because mixing an acidic and basic. In the solid or pure state, these exist as covalent compounds as the hydrogen ions are generated only when these are mixed with water as per arrhenius acid base theory.
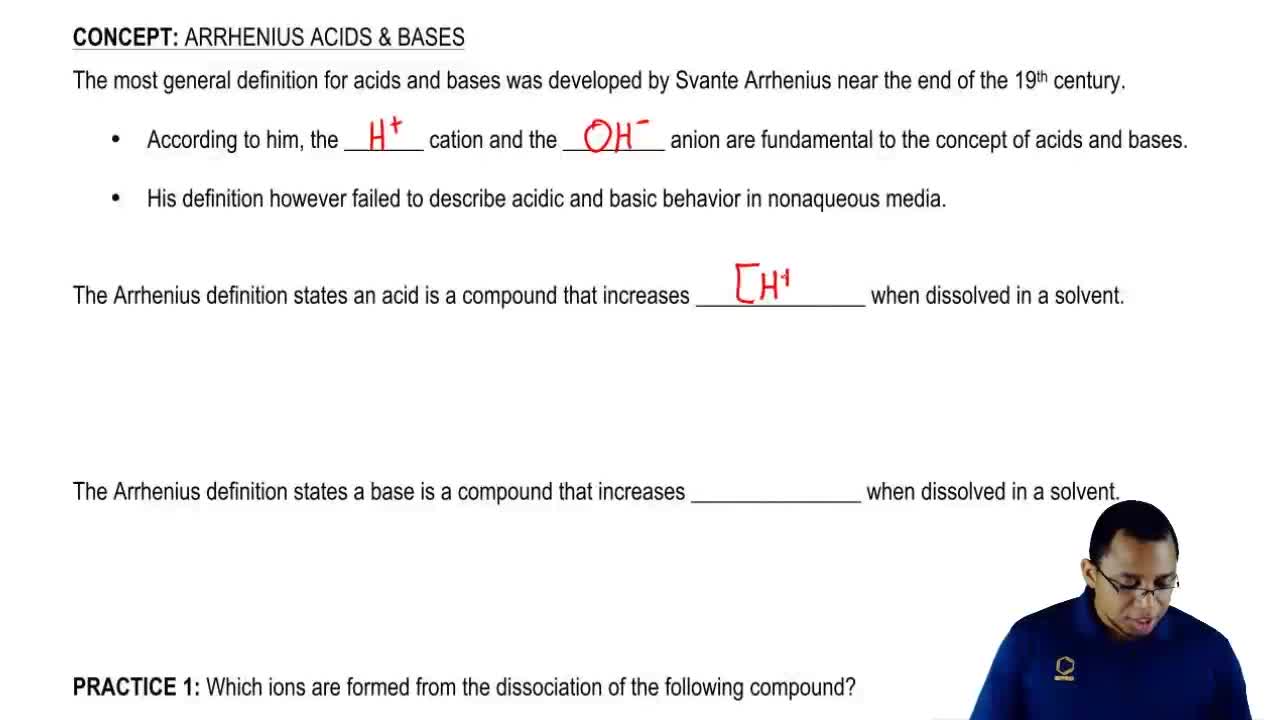 Source: clutchprep.com
Source: clutchprep.com
Which of the following is an arrhenius base? Arrhenius base is a substance that. In our investigation, we looked at the chemical reactions given to us to determine if the compound is an arrhenius acid or not, but we could have come up with these reactions ourselves.
Also Read :