Below is an outline of the periodic table with a number of elements represented only by the letters a to i is shown. The first ionization energy varies in a predictable way across the periodic table.
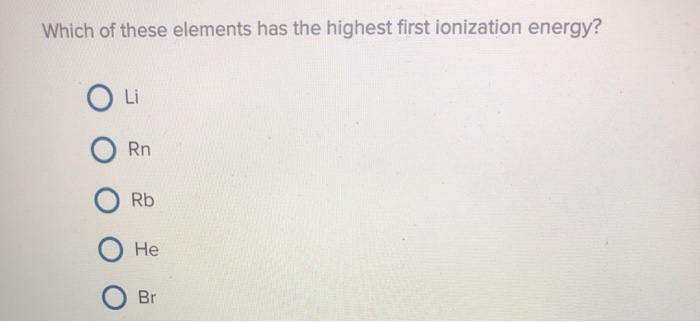
Which Of These Elements Has The Highest First Ionization Energy. It is because of the shielding effect that the ionization energy decreases from top to bottom within a group. The energy decrease and the atom is more reactive. There is a general trend for ionization energy within a period of the periodic table. Element y has the highest first ionisation energy and the lowest melting point of these three elements.
 Solved Which Of These Elements Has The Highest First | Chegg.com From chegg.com
Solved Which Of These Elements Has The Highest First | Chegg.com From chegg.com
Related Post Solved Which Of These Elements Has The Highest First | Chegg.com :
Rank these elements according to first ionization energy (highest to lowest) : Because h e is the smallest atom, and because helium has the greatest effective nuclear charge. Thus, helium has the largest first ionization energy, while. From this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of helium and neon).
Hence, the carbon has the highest ionization energy as it belongs to the rightmost in the periodic table.
Which element in each pair has the larger first ionization energy? Which of the following elements has the highest first ionization energy? Element y has the highest first ionisation energy and the lowest melting point of these three elements. Asked aug 1, 2019 in chemistry by casey. • with ionization energy, energy was added to the atom to remove an electron (making a cation). The highest first ionization energy is undoubtedly that of h e.

Since magnesium and phosphorus are on the same period (period 3), it can be deduced from the explanation above that phosphorus has a greater first ionization energy than magnesium because phosphorus (5 valence electrons) has more electrons in it’s outermost shell than magnesium (2 valence electrons). Given what i have said, you should be able to determine the order. Element y has the highest first ionisation energy and the lowest melting point of these three elements.
 Source: numerade.com
Source: numerade.com
What kind of element has high ionization energy? And look at this handy graph: Below is an outline of the periodic table with a number of elements represented only by the letters a to i is shown.

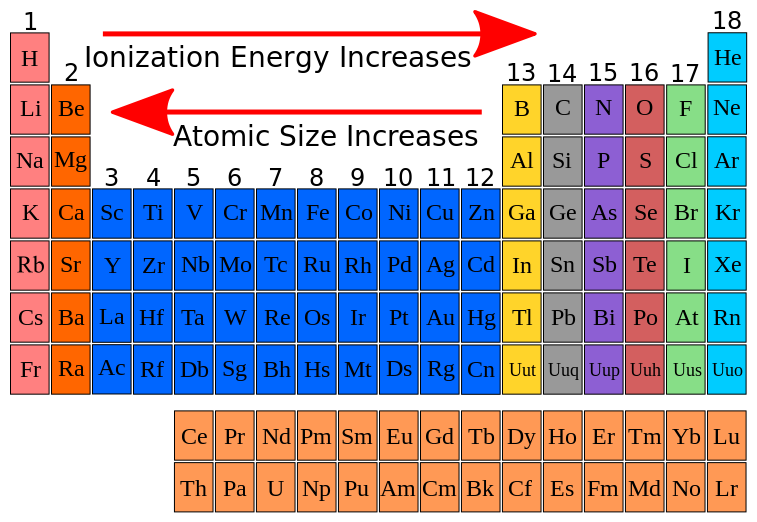
The ionization energy decreases from top to bottom in groups, and increases from left to right across a period. It is because of the shielding effect that the ionization energy decreases from top to bottom within a group. If we were to take a single element then helium is said to have the highest first ionization energy among all the other neutral elements.
 Source: slideplayer.com
Source: slideplayer.com
The trend is that ionization energy increases moving left to right across the table and decreases moving down an element group. Of the following, which element has the highest first ionization energy? The trend of the ionization energies of the elements can be observed in the periodic table:
 Source: chegg.com
Source: chegg.com
Which group of elements has the highest ionization energy? Rank these elements according to first ionization energy (highest to lowest) : Rank the elements c, o, na, and al in order of decreasing ionization energy (largest first, etc.).
 Source: angelo.edu
Source: angelo.edu
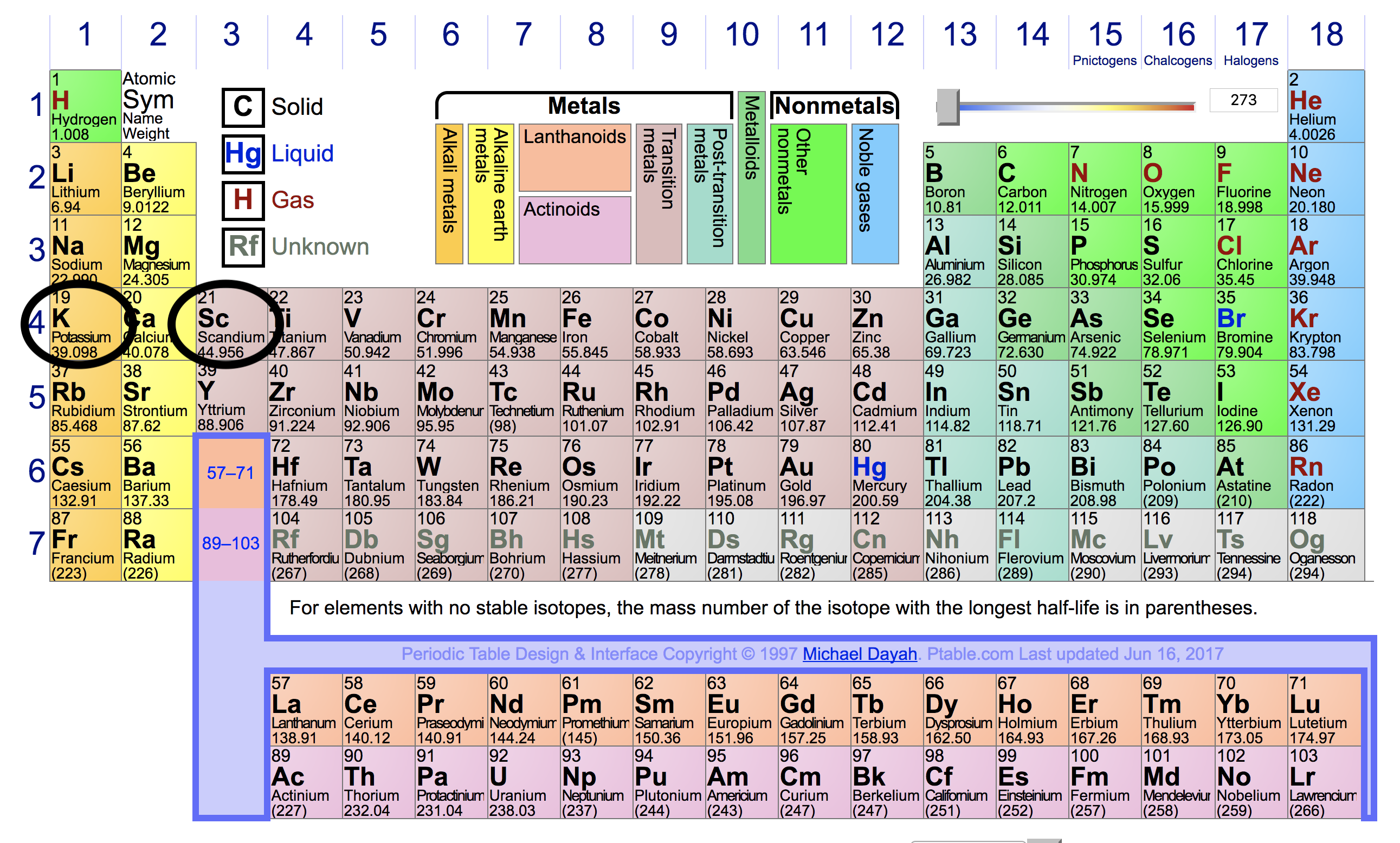
The energy decrease and the atom is more reactive. K, ca, ga, ge, as, se, br and kr let’s check their location in the periodic table and see how they fall in the. The ionization potential is said to be 24.5874 ev.

K, ca, ga, ge, as, se, br and kr let’s check their location in the periodic table and see how they fall in the. 119 rows for chemistry students and teachers: There is a general trend for ionization energy within a period of the periodic table.

Solved which of these elements has the highest first | chegg.com. Because we are physical scientists, while we can make predictions we should look at actual figures to check that we are not talking out of our posteriors. The first ionization energy varies in a predictable way across the periodic table.
 Source: slidetodoc.com
Source: slidetodoc.com
• with ionization energy, energy was added to the atom to remove an electron (making a cation). K, ca, ga, ge, as, se, br and kr let’s check their location in the periodic table and see how they fall in the. If we were to take a single element then helium is said to have the highest first ionization energy among all the other neutral elements.

Which element has the greatest first ionization energy brainly? The first ionization energy varies in a predictable way across the periodic table. Which of these elements has the highest first ionization energy?
 Source: clutchprep.com
Source: clutchprep.com
Thus, helium has the largest first ionization energy, while. Heliumthe ionization energy decreases from top to bottom in groups, and increases from left to right across a period. Thus, helium has the largest first ionization energy, while francium has one of the lowest.
 Source: socratic.org
Source: socratic.org
The trend of the ionization energies of the elements can be observed in the periodic table: The first ionization energy is the amount of energy needed to remove the first electron from the outer shell of an atom or ion. Rank these elements according to first ionization energy (highest to lowest) :
 Source: socratic.org
Source: socratic.org
The ionization energy decreases from top to bottom in groups, and increases from left to right across a period. • with electron affinity, energy is released when an atom gains an electron (making an anion). Sodium has the larger first ionization energy and in the second pair, phosphorus has the largest first ionization energy.
 Source: angelo.edu
Source: angelo.edu
Thus, helium has the largest first ionization energy, while. Therefore the element with the highest 1st ionization should be helium. Consecutive elements x, y and z are in period 3 of the periodic table.
 Source: quizlet.com
Source: quizlet.com
Click to see full answer. Because h e is the smallest atom, and because helium has the greatest effective nuclear charge. Which of these elements has the highest first ionization energy?
 Source: clutchprep.com
Source: clutchprep.com
So, carbon has the highest first ionization energy. Use these letters when answering this question. So from highest to lowest is li>na>k>rb.
 Source: chegg.com
Source: chegg.com
The ionization energy decreases from top to bottom in groups, and increases from left to right across a period. The tabular chart on the right is arranged by. The highest first ionization energy is undoubtedly that of h e.
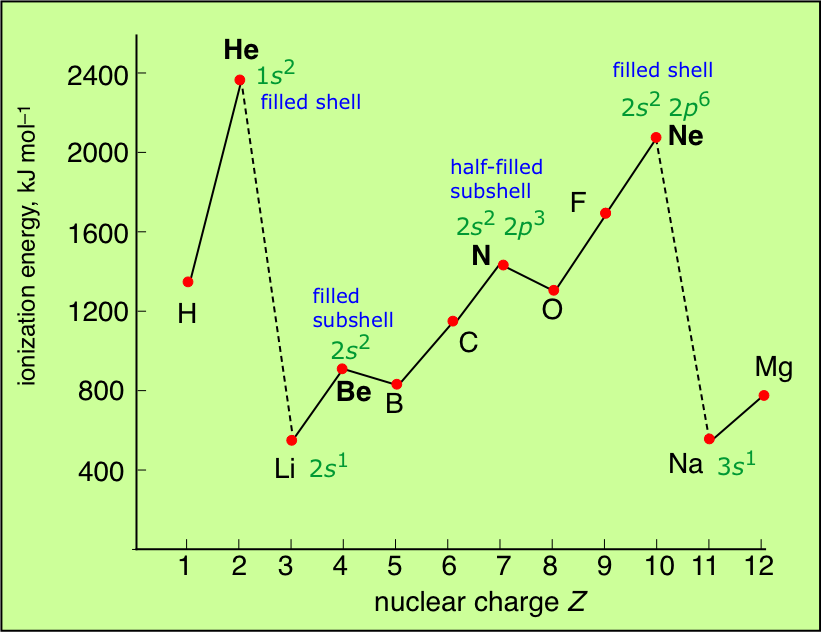 Source: socratic.org
Source: socratic.org
Therefore the element with the highest 1st ionization should be helium. Which element has the greatest first ionization energy brainly? Which element in each pair has the larger first ionization energy?
 Source: numerade.com
Source: numerade.com
Solved which of these elements has the highest first | chegg.com. So from highest to lowest is li>na>k>rb. • opposite of ionization energy.
 Source: youtube.com
Source: youtube.com
Ionization energy is the energy required to remove an electron from a gaseous atom or ion. If we were to take a single element then helium is said to have the highest first ionization energy among all the other neutral elements. So, carbon has the highest first ionization energy.
Also Read :