A substance that increase the amount of hydrogen ions present. Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield electrically charged atoms or molecules, called ions, one of which is a hydrogen ion (h +), and that bases ionize in water to yield hydroxide ions (oh −).
Which Of The Following Substances Is An Arrhenius Acid. For example, consider the dissociation of an acid as follows: They have a sour taste. A) h 2 so 4. What is arrhenius acid base theory?

Related Post Solved An Arrhenius Acid Is Best Defined As A When | Chegg.com :
Which of the following is an arrhenius acid? Which characteristic best identifies an arrhenius… according to the arrhenius concept, if naoh were… a0.50 m solution of an acid ha has ph = 2.24. The general properties of acid and base A) h2so4 b) lioh c) nh2ch3 d) ch3ccl3 e) nh3.
A substance that increase the amount of hydrogen ions present.
Simply put, a proton donor. The general properties of acid and base Which of the following is a. Recall that according to the arrhenius definition: An arrhenius acid increases the concentration of h+ ion when dissolved. Click again to see term 👆.
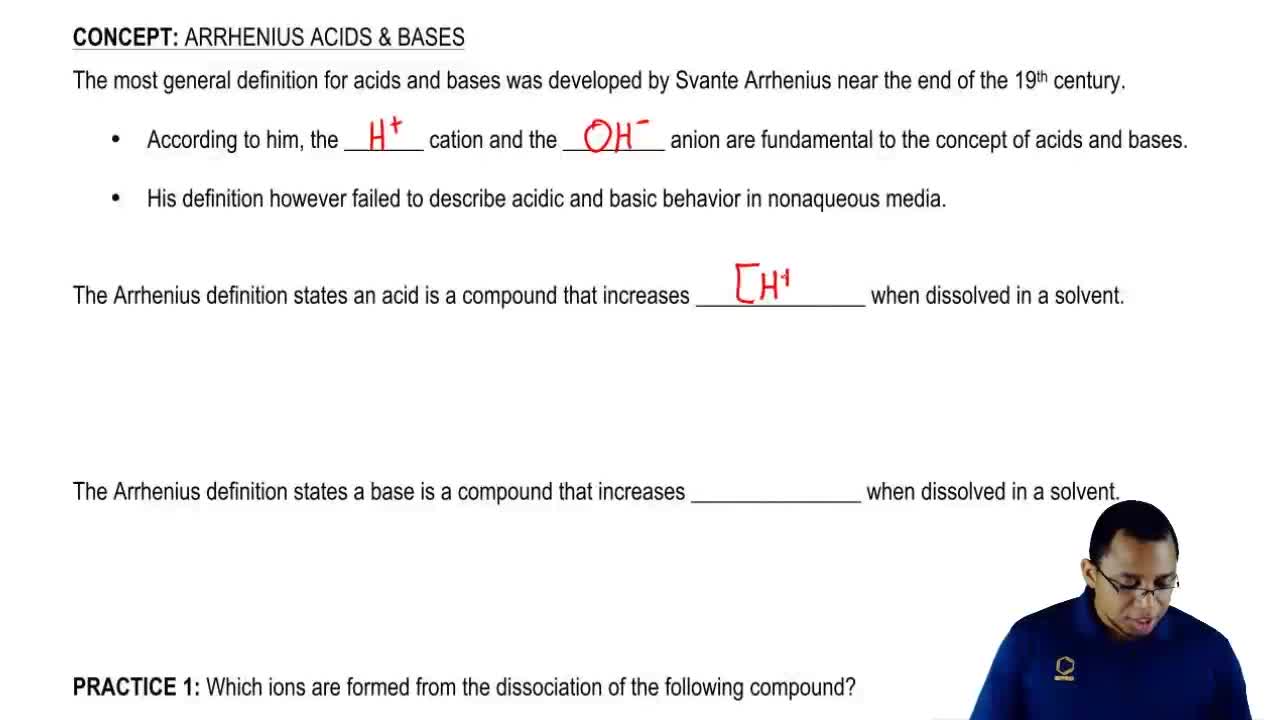 Source: clutchprep.com
Source: clutchprep.com
(a) has a hydrogen atom. An arrhenius acid is best defined as a substance that dissociates in water to produce aqueous hydrogen ions. It dissociates in water, giving and eventually, ions.
 Source: slideplayer.com
Source: slideplayer.com
Which of the following is an arrhenius acid? Means all arrhenius acids are also bronsted acids. Arrhenius theory is only applicable only in aqueous solution, for example, according to the arrhenius theory, hcl is an acid in the aqueous solution but not in benzene, even though it donates ${{\text{h}}^{\text{ + }}}$ ion to the benzene.

The trick to recognizing an arrhenius acid is to look for a molecule that starts with an h, and typically contains an oxygen or halogen. Please mark as brainlist answer. Tap card to see definition 👆.
 Source: chegg.com
Source: chegg.com
These protons go on to form hydronium. A) h 2 so 4. The general properties of acid and base
 Source: slideplayer.com
Source: slideplayer.com
Which of the following statements concerning arrhenius acids and arrhenius bases is incorrect? C) dissociation is the process by which arrhenius acids produce h+ ions in solution. These protons go on to form hydronium.
 Source: study.com
Source: study.com
Please mark as brainlist answer. Common examples of arrhenius acids include: When dissolved in water, which of the following compounds is an arrhenius acid?
 Source: slideserve.com
Source: slideserve.com
Arrhenius acids include sulfuric acid (h2so4), hydrobromic acid (hbr), and nitric acid (hno3). We’re being asked to determine which of the following is an arrhenius acid. Please mark as brainlist answer.

B) in the pure state, arrhenius bases are ionic compounds. An acid is a substance that contains hydrogen and gives h + ions in an aqueous solution. Arrhenius acids are substances which produce hydrogen ions in solution.
 Source: numerade.com
Source: numerade.com
A) h 2 so 4. Also, acids will turn blue litmus paper to red. A substance that increases the amount of hydroxide ions present when it is dissolved in water.

An arrhenius acid is best defined as a substance that dissociates in water to produce aqueous hydrogen ions. C) dissociation is the process by which arrhenius acids produce h+ ions in solution. Arrhenius acids include sulfuric acid (h2so4), hydrobromic acid (hbr), and nitric acid (hno3).
 Source: ethiopialearning.com
Source: ethiopialearning.com
(a) has a hydrogen atom. Characteristic property traditionally associated with arrhenius acid? D) arrhenius bases are also called hydroxide bases.
 Source: slideplayer.com
Source: slideplayer.com
What is arrhenius acid base theory? It dissociates in water, giving and eventually, ions. An arrhenius acid is a chemical compound that a.
 Source: clutchprep.com
Source: clutchprep.com
Also, acids will turn blue litmus paper to red. Which of the following substances is an arrhenius acid oneclass: B) in the pure state, arrhenius bases are ionic compounds.
 Source: chegg.com
Source: chegg.com
It dissolves in water to form the hydrogen ion and chlorine ion: Arrhenius defines acid as a substance which contains h in its formula and produces in water. These protons go on to form hydronium.
 Source: chegg.com
Source: chegg.com
Also, acids will turn blue litmus paper to red. C) dissociation is the process by which arrhenius acids produce h+ ions in solution. A) h2so4 b) lioh c) nh2ch3 d) ch3ccl3 e) nh3.
 Source: chegg.com
Source: chegg.com
Arrhenius concept of acids and bases. The swedish scientist svante august arrhenius defined acids as substances that increase the h + ion concentration of water when dissolved in it. An acid is a substance that contains hydrogen and gives h + ions in an aqueous solution.
 Source: clutchprep.com
Source: clutchprep.com
Arrhenius theory is only applicable only in aqueous solution, for example, according to the arrhenius theory, hcl is an acid in the aqueous solution but not in benzene, even though it donates ${{\text{h}}^{\text{ + }}}$ ion to the benzene. Also, acids will turn blue litmus paper to red. An arrhenius acid increases the concentration of h+ ion when dissolved.
 Source: chegg.com
Source: chegg.com
Recall that according to the arrhenius definition: Also, acids will turn blue litmus paper to red. A) in the pure state, arrhenius acids are covalent compounds.
 Source: homeworklib.com
Source: homeworklib.com
The general properties of acid and base A) h 2 so 4. Also, acids will turn blue litmus paper to red.
 Source: chegg.com
Source: chegg.com
A) strong base b) weak base c) strong acid d) weak acid. For example, consider the dissociation of an acid as follows: A) h2so4 b) lioh c) nh2ch3 d) ch3ccl3 e) nh3.
Also Read :





