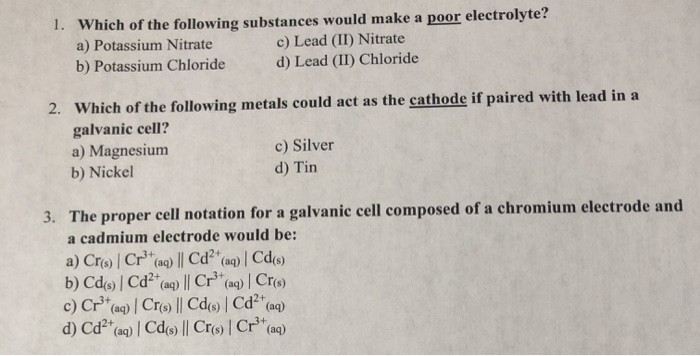
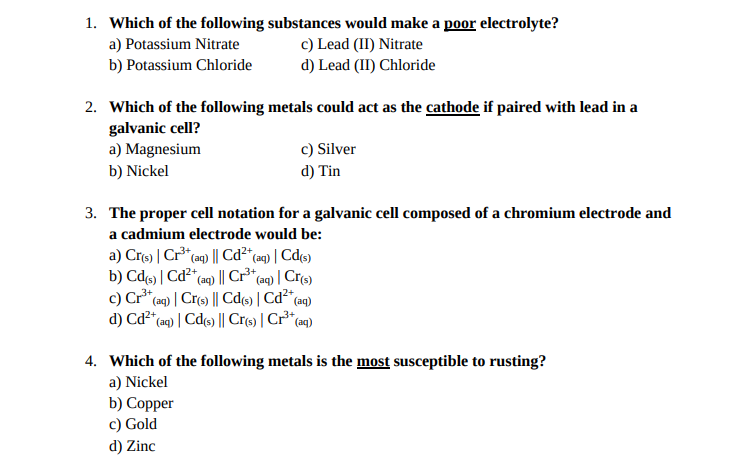
So2cl2(g) ⇔ so2(g) + cl2(g) identify which combination of the following changes will shift the equilibrium to the product side. Which of the following solid metals could act as the anode if paired with cadmium (cd(s)/cd?') in a galvanic cell?
Which Of The Following Substances Acts As An Electrolyte. The lead ions are positively charged and are called cations. (b) molten lead bromide conducts electricity.it is called an electrolyte. A strong electrolyte completely ionizes when dissolved in water to form two or more ions per formula unit of compound. The ionic compounds and strong acid acts as strong electrolyte in water.
 Solved 1. Which Of The Following Substances Would Make A | Chegg.com From chegg.com
Solved 1. Which Of The Following Substances Would Make A | Chegg.com From chegg.com
Related Post Solved 1. Which Of The Following Substances Would Make A | Chegg.com :
Which of the following solid metals could act as the anode if paired with cadmium (cd(s)/cd?�) in a galvanic cell? A) lead c) zinc b) copper d) tin 3. It is composed of lead ions and bromide ions. Which of the following substances acts as an electrolyte?
This is due to the following reasons.
Removal of cl2 i + iv i + ii ii + iii iii + iv Exert osmotic pressure, keeping body fluids in their own compartments. Which of the following substances acts as an electrolyte? An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Acetic acid is a weak electrolyte. An electrolyte is a medium containing ions that is electrically conducting through the movement of ions, but not conducting electrons.
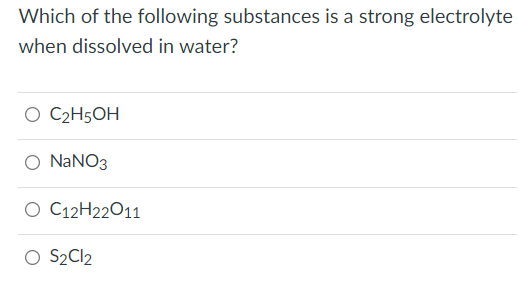 Source: bartleby.com
Source: bartleby.com
An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. Cacl2, hno3, c2h5oh (ethanol), nitric acid, hno3, is a common strong acid (“common strong acids and bases” table), and therefore is a strong electrolyte. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity.
 Source: chegg.com
Source: chegg.com
An electrolyte is a substance that produces ions when dissolved in water. Which of the following solid metals could act as the anode if paired with cadmium (cd(s)/cd?�) in a galvanic cell? Strong acids and ionic compounds are strong electrolytes, while weak acids and weak
It is composed of lead ions and bromide ions. And as this solution has free ions, it conducts electricity. So2cl2(g) ⇔ so2(g) + cl2(g) identify which combination of the following changes will shift the equilibrium to the product side.
 Source: doubtnut.com
Source: doubtnut.com
Removal of cl2 i + iv i + ii ii + iii iii + iv The lead ions are positively charged and are called cations. So2cl2(g) ⇔ so2(g) + cl2(g) identify which combination of the following changes will shift the equilibrium to the product side.

Which of the following solid metals could act as the anode if paired with cadmium (cd(s)/cd?�) in a galvanic cell? An electrolyte is a substance that produces ions when dissolved in water. A) lead (ii) nitrate c) silver bromide b) potassium chloride d) ammonium phosphate 2.
 Source: circuitglobe.com
Source: circuitglobe.com
So this is an electrolyte. An ionic compound for example, sodium chloride dissolved in water is called an electrolyte because it conducts electricity. The substances whose aqueous solutions allow the passage of electric current and are chemically decomposed, are termed electrolytes.

A) sodium sulfate b) ethyl alcohol c) oxygen d) sucrose (b) molten lead bromide conducts electricity.it is called an electrolyte. It can cause very severe chemical burns on skin.

Doesn’t dissociate completely in water; It is composed of lead ions and bromide ions. So2cl2(g) ⇔ so2(g) + cl2(g) identify which combination of the following changes will shift the equilibrium to the product side.

A weak electrolyte ionizes to only a small extent, producing much less than two ions per formula unit of dissolved compound. A weak electrolyte ionizes to only a small extent, producing much less than two ions per formula unit of dissolved compound. Exert osmotic pressure, keeping body fluids in their own compartments.

It can cause very severe chemical burns on skin. It�s metal in bromide, obviously is a allied. Electrolytic substances are classified as strong or weak according to how readily they dissociate into conducting ions.
 Source: chegg.com
Source: chegg.com
Those that follow the rules of being soluble in solubility rules. So2cl2(g) ⇔ so2(g) + cl2(g) identify which combination of the following changes will shift the equilibrium to the product side. A) sodium sulfate b) ethyl alcohol c) oxygen d) sucrose
So2cl2(g) ⇔ so2(g) + cl2(g) identify which combination of the following changes will shift the equilibrium to the product side. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water.upon dissolving, the substance separates into cations and anions, which disperse uniformly through the solvent. Which one of the following would act like a strong electrolyte in an aqueous solution?
 Source: chegg.com
Source: chegg.com
A weak electrolyte ionizes to only a small extent, producing much less than two ions per formula unit of dissolved compound. Exert osmotic pressure, keeping body fluids in their own compartments. The ionic compounds and strong acid acts as strong electrolyte in water.
The second one is some large hydrocarbon, and these. Acetic acid is a weak electrolyte. (b) molten lead bromide conducts electricity.it is called an electrolyte.
 Source: chegg.com
Source: chegg.com
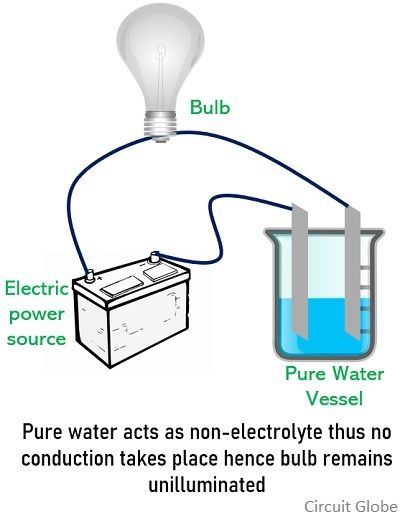
It can cause very severe chemical burns on skin. Pure water is a very weak electrolyte. The lead ions are positively charged and are called cations.
 Source: chegg.com
Source: chegg.com
The lead ions are positively charged and are called cations. It�s metal in bromide, obviously is a allied. A) lead (ii) nitrate c) silver bromide b) potassium chloride d) ammonium phosphate 2.
 Source: doubtnut.com
Source: doubtnut.com
The second one is some large hydrocarbon, and these. Which of the following substances acts as an electrolyte? An electrolyte is a medium containing ions that is electrically conducting through the movement of ions, but not conducting electrons.
 Source: chegg.com
Source: chegg.com
Okay, chapter eight section problem forty asks us to classify each calm bone as a strong electrolyte or a non electrolyte became a is magnesium bromide, and magnesium is an alkaline earth metal. Which of the following substances acts as an electrolyte? Consider the following system at equilibrium:
 Source: doubtnut.com
Source: doubtnut.com
Urea, benzene, sugar, ethanol, chloroform , ether etc. Those that follow the rules of being soluble in solubility rules. It�s metal in bromide, obviously is a allied.

Electrolytic substances are classified as strong or weak according to how readily they dissociate into conducting ions. Which of the following is an electrolyte when dissolved in distilled water? Pure water is a very weak electrolyte.
Also Read :





