A steel rod of diameter 10 mm is clamped firmly at each end when its temperature is so that it cannot contract on cooling the tension in the rod at is approximately . C)a catalyst is consumed in a chemical reaction.
Which Of The Following Statements About A Catalyst Is True. A catalyst accelerates a reaction by lowering the; Which of the following statements about a catalyst is true? It involves breaking down more useful compounds into smaller and more useful ones catalytic cracking involves a free radical mechanism catalytic cracking involves heterolytic fission. A) a catalyst changes the position of the equilibrium in a reaction.

Related Post Solved Which Of The Following Statements About A Catalyst | Chegg.com :
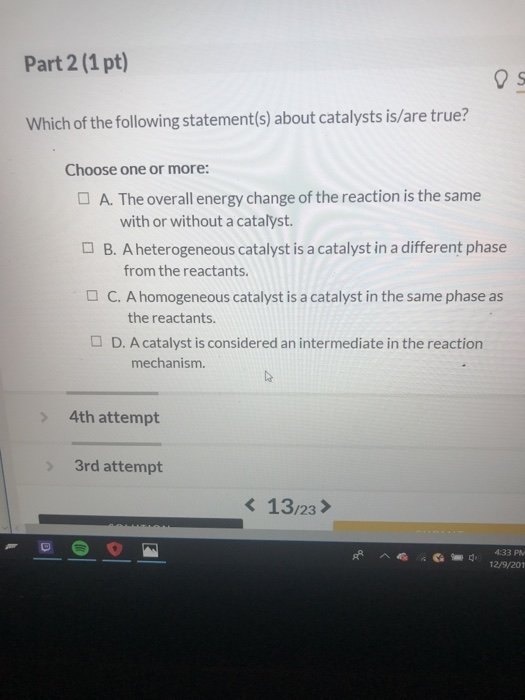
Which of the following statements about a catalyst is true? B) a catalyst increases the temperature of a reaction. So what we need to do is go through and look at what is it that a catalyst does have? So this question is asking about properties of catalysts and what they do in aiding with reactions, chemical reactions and the actual gist of the reaction is which one of the following is not something that a catalyst would have.
Which of the following statements is true?
A catalyst may be used in the solid, liquid or gaseous phase. A catalyst alters the position of equilibrium. The rate of all chemical reactions doubles for every 10 o c increase in temperature. Activation energy is thus affected by the use of catalysts, therefore the answer is option d, ea. Which of the following statements about a catalyst is/are true? In a first order reaction 80% of the reactant at an instant was reduced to 8% in 4606 seconds.
 Source: chegg.com
Source: chegg.com
Which of the following statements about a catalyst is/are true? O catalysts cause a system at equilibrium to shift towards the products. A catalyst makes the reaction faster by providing an alternative pathway with a lower activation energy.
 Source: chegg.com
Source: chegg.com
The rate constant of the reaction is. It is future rather than past centered. A catalyst accelerates a reaction by lowering the;
 Source: chegg.com
Source: chegg.com
All of the answers are correct. Which of the following statements regarding catalyst is not true ? It is future rather than past centered.
 Source: chegg.com
Source: chegg.com
A catalyst can be consumed in a reaction as long as it is regenerated.e. Catalysts increase the temperature of a chemical reaction. Which of the following statements about a catalyst is true?
 Source: toppr.com
Source: toppr.com
Which one of the following statements regarding catalysis is true ? Xiangling�s charged attack has a. T 1 / 2 for a first order reaction is 14.26 m i n.
 Source: doubtnut.com
Source: doubtnut.com
A catalyst accelerates the reaction by bringing down the energy of activation. A catalyst speeds up the reaction by decreasing the frequency factor d. Which of the following statements about catalysis are true?
 Source: chegg.com
Source: chegg.com
When xiangling uses her elemental skill, guoba will deal aoe pyro dmg 4 times. A steel rod of diameter 10 mm is clamped firmly at each end when its temperature is so that it cannot contract on cooling the tension in the rod at is approximately . Catalyst does not change the equilibrium constant of a reverible reaction, rather it helps in attaining th equilibrium faster because it catalyes the forward as well as the backward reactions to the same exent so that the equilibrium state remains the same extent so but is attained erlier.
 Source: bartleby.com
Source: bartleby.com
Which of the following statements about a catalyst is true? A)a catalyst changes the position of the equilibrium in a reaction. Ic] the concentration of the products equals that of reactants and is constant at equilibrium [d] an endothermic reaction.
 Source: toppr.com
Source: toppr.com
A catalyst does not change the energy of the reactants or the products.c. Which of the following statements is true about catalytic coaching? So this question is asking about properties of catalysts and what they do in aiding with reactions, chemical reactions and the actual gist of the reaction is which one of the following is not something that a catalyst would have.
 Source: chegg.com
Source: chegg.com
A catalyst speeds up the reaction by decreasing the frequency factor d. Which of the following statements is true? A catalyst makes the equilibrium constant of the reaction more favourable for the forward reaction.
 Source: chegg.com
Source: chegg.com
When xiangling uses her elemental skill, guoba will deal aoe pyro dmg 4 times. So what we need to do is go through and look at what is it that a catalyst does have? What is not true for a catalyst?
 Source: chegg.com
Source: chegg.com
A catalyst can convert an endothermic reaction into an exothermic reaction. Catalysts increase the temperature of a chemical reaction. Add your answer and earn points.
 Source: toppr.com
Source: toppr.com
- because many biologically important reactions are very slow, living organisms rely on a class of catalysts called enzymes to increase the rate of biochemical reactions. All of the answers are correct. A) a catalyst changes the position of the equilibrium in a reaction.
 Source: chegg.com
Source: chegg.com
Add your answer and earn points. What are the qualities or what is that a catalyst will dio first. Which of the following statements is true about catalytic coaching?
 Source: chegg.com
Source: chegg.com
Which of the following statements is true? Which of the following statements about a catalyst is true? Which of the following statements is true?

Add your answer and earn points. 7 inches of a foot. Which of the following statements about catalysts is not true?a.
 Source: chegg.com
Source: chegg.com
Which one of the following statements about catalysts is true? A) a catalyst accelerates a reaction by changing the amount of reactant and product at equilibrium. D) a catalyst accelerates a reaction by lowering the equilibrium constant.
 Source: clutchprep.com
Source: clutchprep.com
A catalyst does not take part in the reaction. O catalysts decrease the rate of the reaction. Which of the following statements regarding catalyst is not true ?
Which one of the following statements about catalytic cracking is not true? A catalyst speeds up the reaction by decreasing the frequency factor d. B1 when two opposing processes are proceeding at identical rates, the system is at 2.
![Solved:which Of The Following Statements . About Catalysts Is True? [Select All That Apply] A Catalyst Can Provide Different Reaction Pathway With Lower Activation Energy Acatalyst Stabilizes The Product Of The Rcaction Solved:which Of The Following Statements . About Catalysts Is True? [Select All That Apply] A Catalyst Can Provide Different Reaction Pathway With Lower Activation Energy Acatalyst Stabilizes The Product Of The Rcaction](https://cdn.numerade.com/ask_images/a05c12f7cb0e47ad8ce6f70b4c1fdc90.jpg) Source: numerade.com
Source: numerade.com
O catalysts increase the activation energy of a reaction. Activation energy is thus affected by the use of catalysts, therefore the answer is option d, ea. A catalyst can be consumed in a reaction as long as it is regenerated.e.
Also Read :