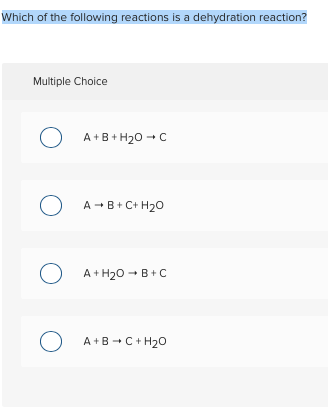
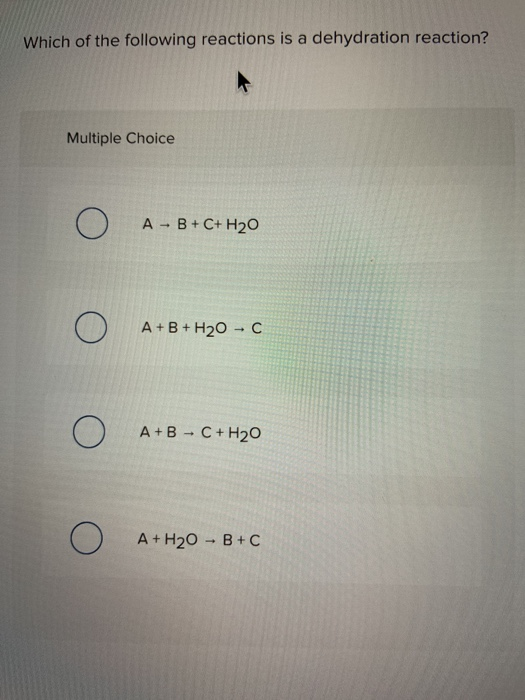
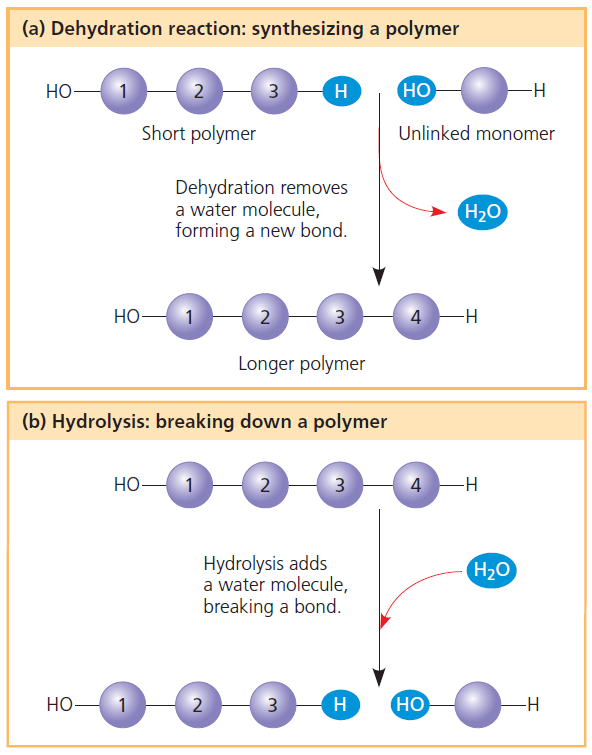
Dehydration synthesis is the process of joining two molecules, or compounds, together following the removal of water. 10) identify the following reaction:
Which Of The Following Reactions Is A Dehydration Reaction. Dehydration reactions split water molecules and add hydroxyl groups to polymers, and hydrolysis reactions remove hydroxyl groups from polymers. C + h 2 o à a + b o An oxidative decarboxylation, a dehydration, and a condensation d. Notice that c is the final product with no a or b remaining as a residue.
 Alcohol Reactivity From www2.chemistry.msu.edu
Alcohol Reactivity From www2.chemistry.msu.edu
Related Post Alcohol Reactivity :
In chemistry, a dehydration reaction is a conversion that involves the loss of water from the reacting molecule or ion.dehydration reactions are common processes, the reverse of a hydration reaction.common dehydrating agents used in organic synthesis include sulfuric acid and alumina. At room temperature, the reaction does not occur. A + b → c. Which one is a hydrolysis reaction?
Polymers are formed via dehydration reactions by molecules.
The reactions in which succinate is converted to oxaloacetate are, in order a. A + b → c. It is a kind of condensation reaction in which water molecule eliminate with the addition of. Assembling molecules in either carbonyl or carboxyl functional groups assembling molecules in either hydroxyl or carboxyl functional groups dissembling molecules in carboxyl functional groups dissembling molecules in hydroxyl. Note that the reaction has three steps: A) synthesis of atp from adp and ℗i b) a dehydration reaction between two monosaccharides to produce a disaccharide c) formation of a peptide bond d) hydrolysis of glycogen to release glucose monomers

These reactions are known as dehydrogenation or dehydration of alcohols. Which of the following is an exergonic reaction? A condensation, a dehydration, and an oxidative decarboxylation
 Source: chegg.com
Source: chegg.com
Hcl + nahco3 → nacl + h2co3 a) dehydration synthesis. For example, two monomers may react where a hydrogen (h) from one monomer binds to a hydroxyl group (oh) from the other monomer to form a dimer and a water molecule (h 2 o). The 2nd is slow and endothermic.
 Source: study.com
Source: study.com
In chemistry, a dehydration reaction is a conversion that involves the loss of water from the reacting molecule or ion.dehydration reactions are common processes, the reverse of a hydration reaction.common dehydrating agents used in organic synthesis include sulfuric acid and alumina. For example, if two reactants are combined where a hydrogen from one reactant binds to a hydroxyl group from the other reactant, it can produce a dimer and a water molecule. Explore the definition and examples of a dehydration reaction and discover the difference between.
 Source: youtube.com
Source: youtube.com
Which of the following best describes a dehydration reaction? When you see the word dehydration, the first thing that may come to mind is �losing water� or �lacking water.dehydration synthesis is classified as a type of chemical reaction. C + h 2 o à a + b o
 Source: chegg.com
Source: chegg.com
A dehydration reaction is a chemical reaction between two compounds where one of the products is water. Which of the following chemical equations describes a dehydration reaction? 9) identify the following reaction:
 Source: researchgate.net
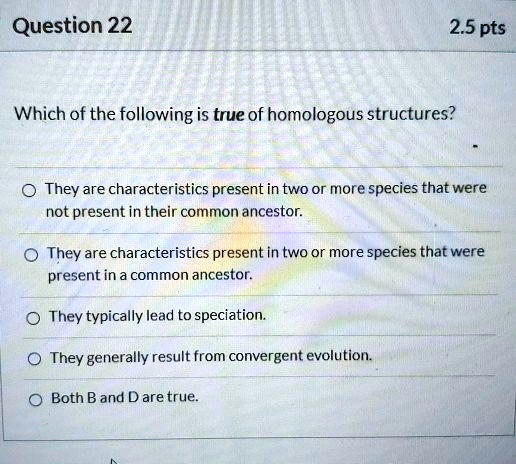
Source: researchgate.net
Carbohydrates, proteins, and nucleic acids are built up and broken down via these types of reactions, although the monomers involved are different in each case. An elimination reaction occurs when a reactant is broken up into two products. During a condensation reaction, two molecules are condensed and water is lost to form a large molecule.
 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
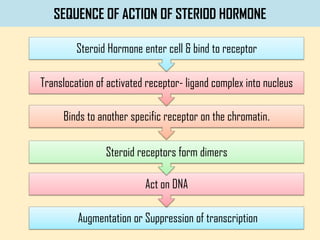
This is the same exact process that occurs during a dehydration synthesis. Which of the following is an exergonic reaction? Lactose + h2o → glucose + galactose a) dehydration synthesis reaction b) hydrolysis reaction c) exchange reaction d) reversible reaction e) ionic reaction.
 Source: clutchprep.com
Source: clutchprep.com
Dehydration reactions are common processes, the reverse of a hydration reaction. In chemistry, a dehydration reaction (a.k.a. Assembling molecules in either carbonyl or carboxyl functional groups assembling molecules in either hydroxyl or carboxyl functional groups dissembling molecules in carboxyl functional groups dissembling molecules in hydroxyl.
 Source: chegg.com
Source: chegg.com
A dehydration reaction is a chemical reaction between two compounds where one of the products is water. For example, two monomers may react where a hydrogen (h) from one monomer binds to a hydroxyl group (oh) from the other monomer to form a dimer and a water molecule (h 2 o). Dehydration synthesis is the process of joining two molecules, or compounds, together following the removal of water.
 Source: chegg.com
Source: chegg.com
A dehydration reaction is a chemical reaction between two compounds where one of the products is water. The reactions in which succinate is converted to oxaloacetate are, in order a. A condensation, a dehydration, and an oxidative decarboxylation

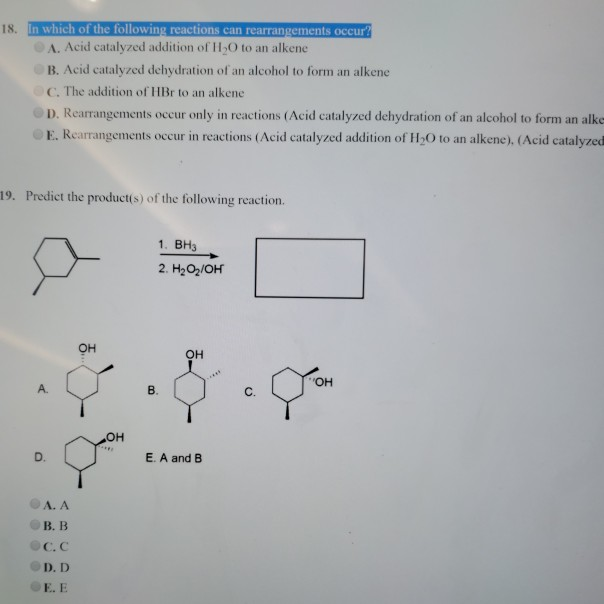
If a, b, and c are molecules and c is a larger molecule than a and b, which of the two reactions is a dehydration reaction? Its rate varies for primary, secondary and tertiary alcohols. Condensation reaction), also known as zimmer’s hydrogenesis, is a conversion that involves the loss of water from the reacting molecule or ion.
 Source: chegg.com
Source: chegg.com
An oxidative decarboxylation, a dehydration, and a condensation d. For example, if two reactants are combined where a hydrogen from one reactant binds to a hydroxyl group from the other reactant, it can produce a dimer and a water molecule. C + h 2 o à a + b o
 Source: sahay.guru
Source: sahay.guru
Which one is a hydrolysis reaction? What is a dehydration condensation reaction? Updated on june 25, 2019.
 Source: chegg.com
Source: chegg.com
- identify the following reaction: Condensation reaction), also known as zimmer’s hydrogenesis, is a conversion that involves the loss of water from the reacting molecule or ion. For example formation of the peptide from amino acids is a dehydration synthesis.
 Source: numerade.com
Source: numerade.com
In an elimination reaction the double bond is formed between the α and β c atoms. Which of the following is an exergonic reaction? A) synthesis of atp from adp and ℗i b) a dehydration reaction between two monosaccharides to produce a disaccharide c) formation of a peptide bond d) hydrolysis of glycogen to release glucose monomers
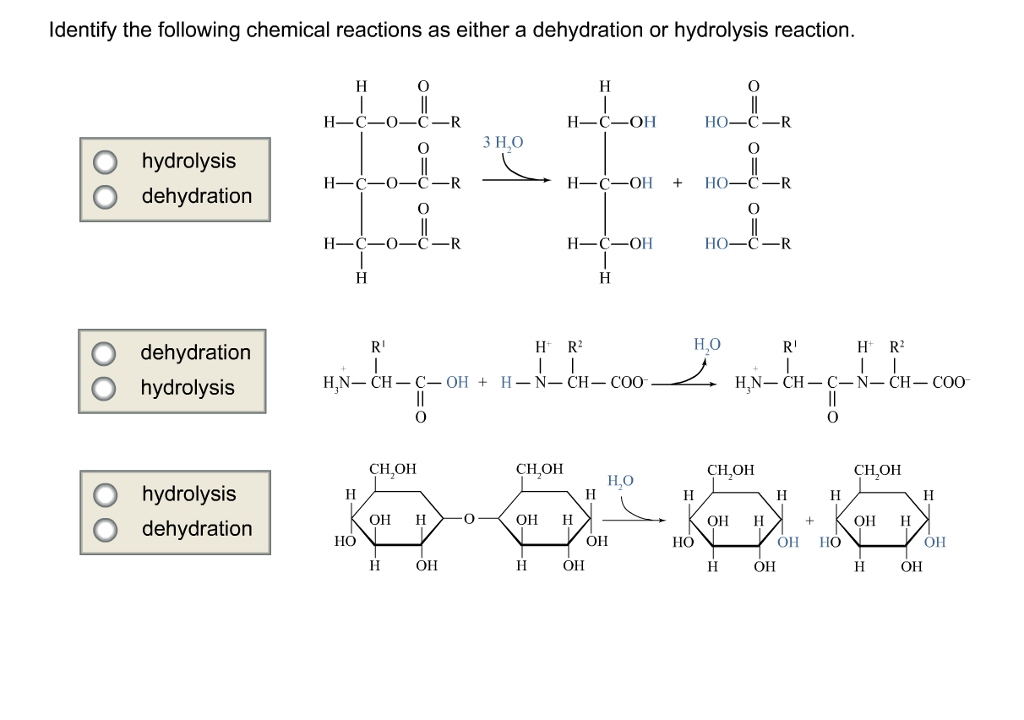 Source: chegg.com
Source: chegg.com
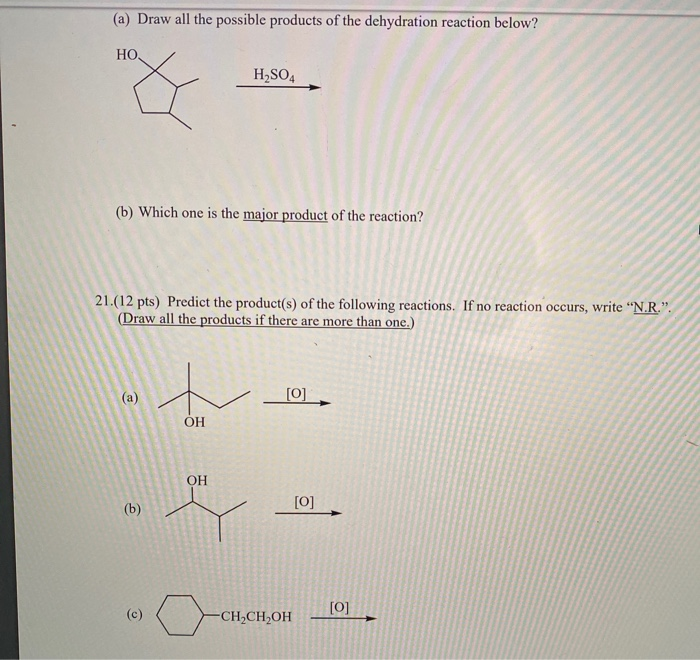
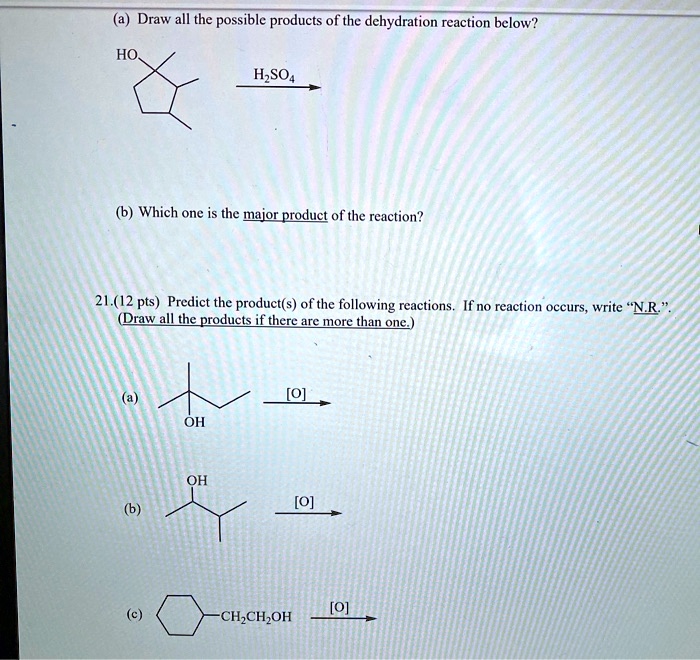
Dehydration synthesis reactions are combination or synthesis reactions which occurs between the same or different monomer units with the elimination of water molecules. For example, two monomers may react where a hydrogen (h) from one monomer binds to a hydroxyl group (oh) from the other monomer to form a dimer and a water molecule (h2o). Give structures of the products you would expect when each of the following alcohols reacts with (a) hcl/zncl 2 (b) hbr (c) socl 2:
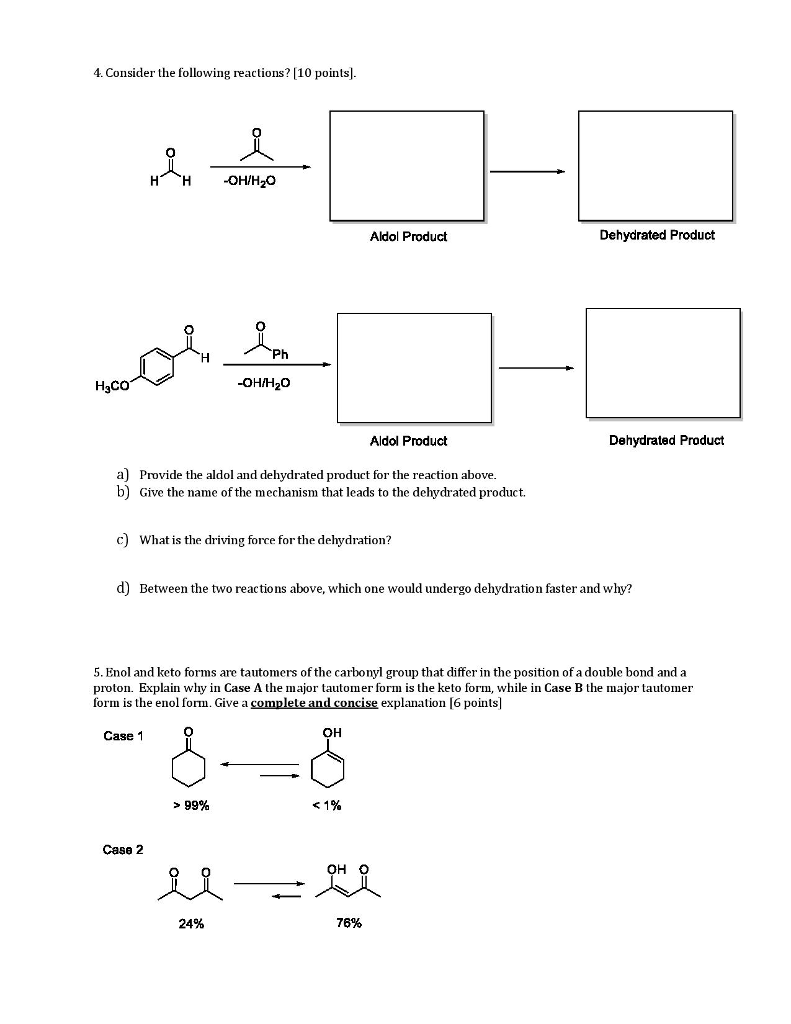 Source: chegg.com
Source: chegg.com
The reactions in which succinate is converted to oxaloacetate are, in order a. Explore the definition and examples of a dehydration reaction and discover the difference between. Assembling molecules in either carbonyl or carboxyl functional groups assembling molecules in either hydroxyl or carboxyl functional groups dissembling molecules in carboxyl functional groups dissembling molecules in hydroxyl.
 Source: slideplayer.com
Source: slideplayer.com
Dehydration synthesis is the process of joining two molecules, or compounds, together following the removal of water. The 1st is fast and endothermic. Assembling molecules in either carbonyl or carboxyl functional groups assembling molecules in either hydroxyl or carboxyl functional groups dissembling molecules in carboxyl functional groups dissembling molecules in hydroxyl.

D) dehydration reactions assemble polymers, and hydrolysis reactions break down polymers. Elimination reactions occur with saturated compounds. A + b à c + h 2 b.
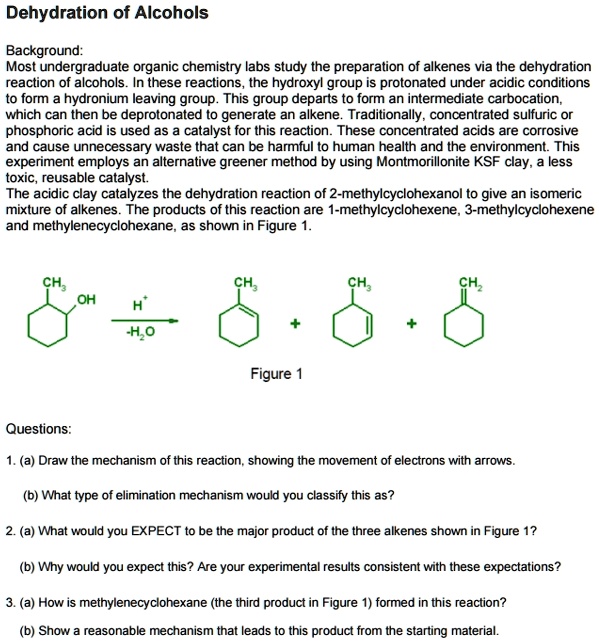 Source: numerade.com
Source: numerade.com
Give structures of the products you would expect when each of the following alcohols reacts with (a) hcl/zncl 2 (b) hbr (c) socl 2: Which of the following best describes a dehydration reaction? For example, if two reactants are combined where a hydrogen from one reactant binds to a hydroxyl group from the other reactant, it can produce a dimer and a water molecule.
Also Read :