(a) the higher the boiling point. So i2 has the strongest forces, and f2 will have the weakest.
Which Of The Following Molecules Has The Highest Boiling Point. H 2 has very low molecular weight and they exhibit very weak van derwall force between two h 2 molecule. Question 17 kcl has the highest boiling point boiling point is in the order kcl>h2o>co2 ( in kcl inter molecular force is ionic bond. On the basis of bonding, the compound which has highest boiling point is sodium chloride. It shows that hi has the higher boiling point.
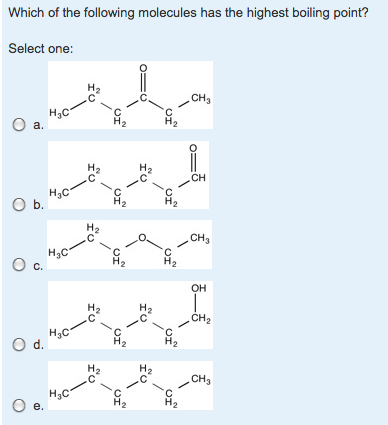
![Solved] Which Of The Following Molecules Has The Highest Boiling Point? Select One: Q A. Ch36H1Ch3 I? | Course Hero](https://www.coursehero.com/qa/attachment/2811568/ “Solved] Which Of The Following Molecules Has The Highest Boiling Point? Select One: Q A. Ch36H1Ch3 I? | Course Hero”) Solved] Which Of The Following Molecules Has The Highest Boiling Point? Select One: Q A. Ch36H1Ch3 I? | Course Hero From coursehero.com
Related Post Solved] Which Of The Following Molecules Has The Highest Boiling Point? Select One: Q A. Ch36H1Ch3 I? | Course Hero :
Which of the following has the highest boiling point? Alcohol are found to have boiling point approximately as 78.37 °c. Straight chain alkyl halides have greater boiling point than their isomers. Methanol has strong hydrogen bonds.
Ionic compounds typically have high.
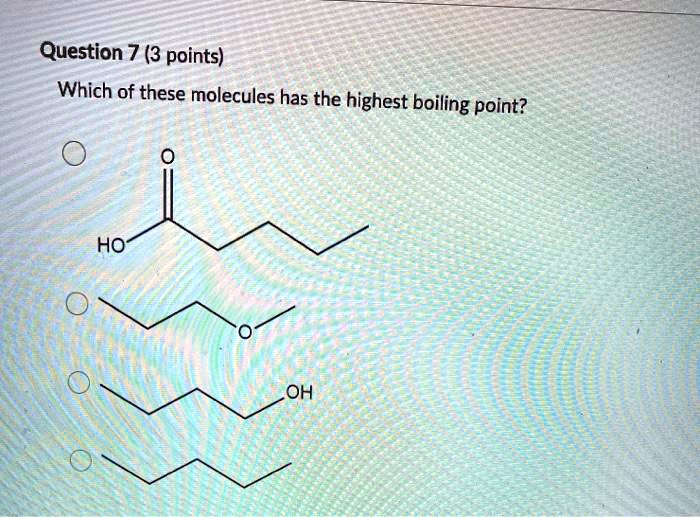
Should have the highest boiling point? Hydrogen bonding is the strongest intermolecular force. He, ne and xe are nobel gases. Therefore, the carboxylic acid that has the highest boiling point is answer choice (e), pentanoic acid. Hence, option (b) is correct. This problem has been solved!
 Source: bartleby.com
Source: bartleby.com
Straight chain alkyl halides have greater boiling point than their isomers. Acetic acid boiling point is. At boiling point heat supplied to substance doesn�t raise the temperature but is used to convert liquid into vapour, boiling point depends on the pressure of the surrounding.
 Source: amp.doubtnut.com
Source: amp.doubtnut.com
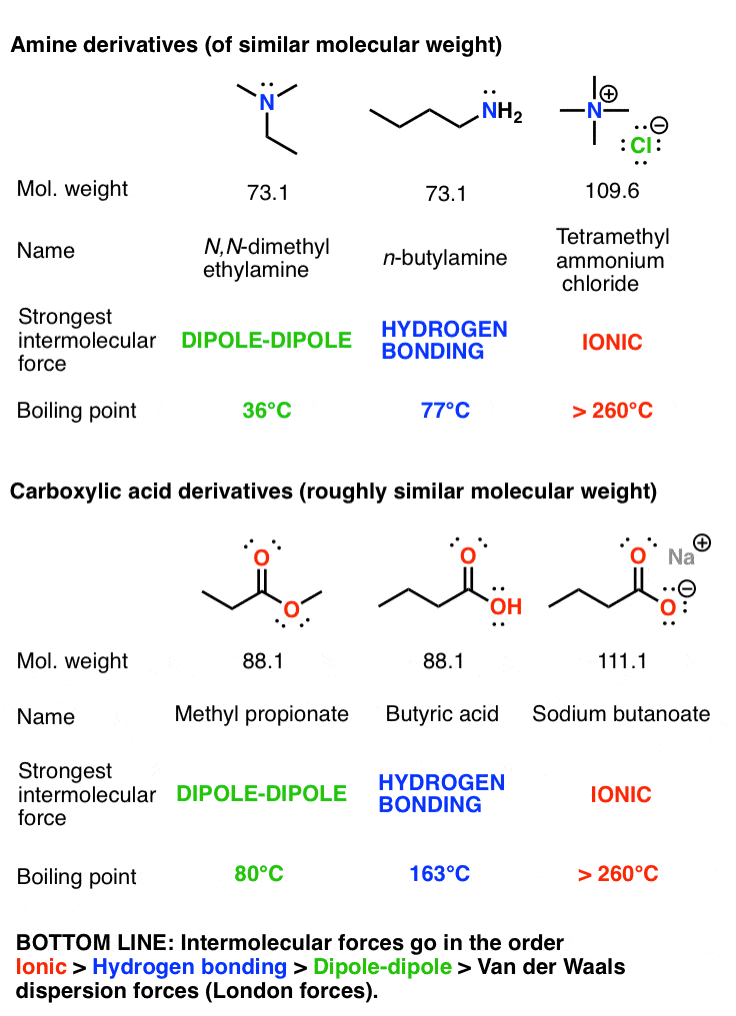
Branching decreases the boiling point. Ch3ch2ch3(44 amu, 0.1 dipole moment) ch3och3(46 amu, 1.3 dipole moment) ch3cl(50 amu, 1.9 dipole moment) ch3cho(44 amu, 2.7 dipole moment) ch3cn(41 amu, 3.9 dipole moment) (b) the lower the boiling point.
 Source: studylib.net
Source: studylib.net
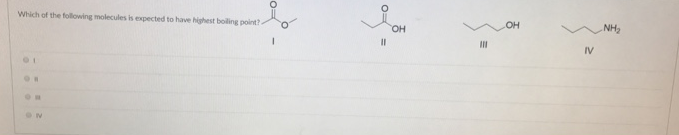
Dimethyl ether, ch_3och_3, is a polar molecule. Therefore, the carboxylic acid that has the highest boiling point is answer choice (e), pentanoic acid. So the one with the highest molar mass will be the highest boiling point.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
It shows that hi has the higher boiling point. (a) the higher the boiling point. Ch4 = nonpolar molecule = london forces = lowest boiling point.
 Source: chegg.com
Source: chegg.com
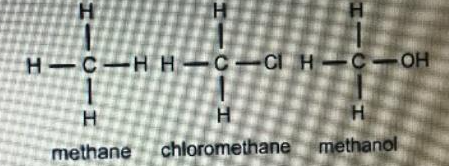
Hence break the bond is very difficult. So, they have lower boiling point than that of methane. This would result in a higher temperature at which boiling would occur.
 Source: chegg.com
Source: chegg.com
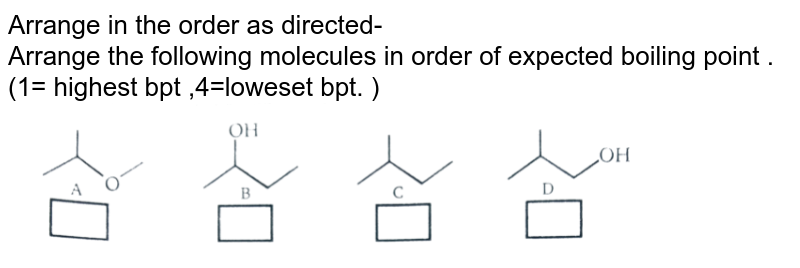
In the dotted box write the iupac name of each of the molecules drawn below. In the dotted box write the iupac name of each of the molecules drawn below. In this case, hcl, hbr and hi all have dipoles, but ldf forces appear to be more important in determining the boiling point
 Source: chegg.com
Source: chegg.com
Q4 rank the following molecule according to their increasing stability. Straight chain alkyl halides have greater boiling point than their isomers. We can see that the largest carboxylic acid from our answer choices is pentanoic acid.
 Source: numerade.com
Source: numerade.com
Q4 rank the following molecule according to their increasing stability. Correspondingly, i2 will have the highest boiling point and f2 will have the lowest boiling point. Arrange the compounds in order of increasing boiling point.
 Source: study.com
Source: study.com
So i2 has the strongest forces, and f2 will have the weakest. Which substance has the highest boiling point? (a) the higher the boiling point.
 Source: studylib.net
Source: studylib.net
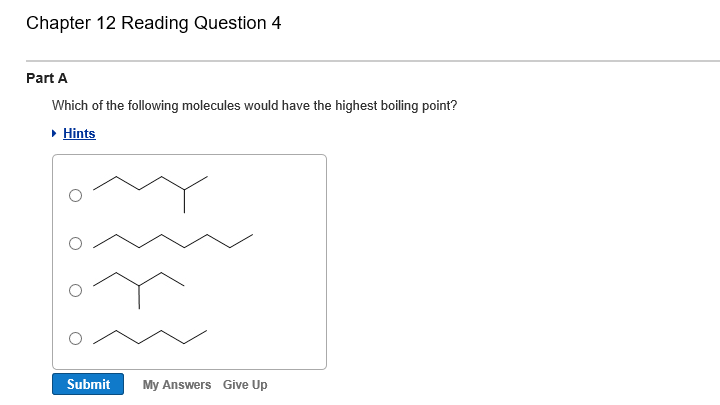
Branching decreases the boiling point. Answer:hf will have the highest boiling point. Bigger molecules will have stronger london dispersion forces.
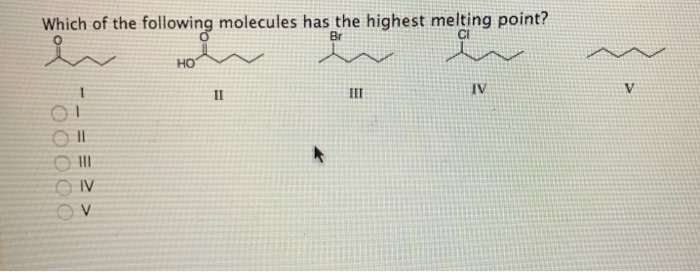
We can see that the largest carboxylic acid from our answer choices is pentanoic acid. F2, cl2, br2, i2 which substance has the highest/lowest melting/boiling point, etc. Acetic acid boiling point is.
 Source: clutchprep.com
Source: clutchprep.com
A)c2cl6 b)c2br6 c)c2h6 d)c2f6 e)c2i6 i know its e, but. (a) ch4 (b) he (c) hf (d) cl2 3. As branching increases boiling point decreases.
 Source: youtube.com
Source: youtube.com
Due to strong intermolecular forces, the molecules of hf will be tightly packed in a lattice. Dimethyl ether, ch_3och_3, is a polar molecule. Alcohol are found to have boiling point approximately as 78.37 °c.
![Solved] Which Of The Following Molecules Has The Highest Boiling Point? Select One: Q A. Ch36H1Ch3 I? | Course Hero](https://www.coursehero.com/qa/attachment/2811568/ “Solved] Which Of The Following Molecules Has The Highest Boiling Point? Select One: Q A. Ch36H1Ch3 I? | Course Hero”) Source: coursehero.com
He, ne and xe are nobel gases. Where 1 is the lowest boiling point and 4 is the highest boiling point. A)c2cl6 b)c2br6 c)c2h6 d)c2f6 e)c2i6 i know its e, but.
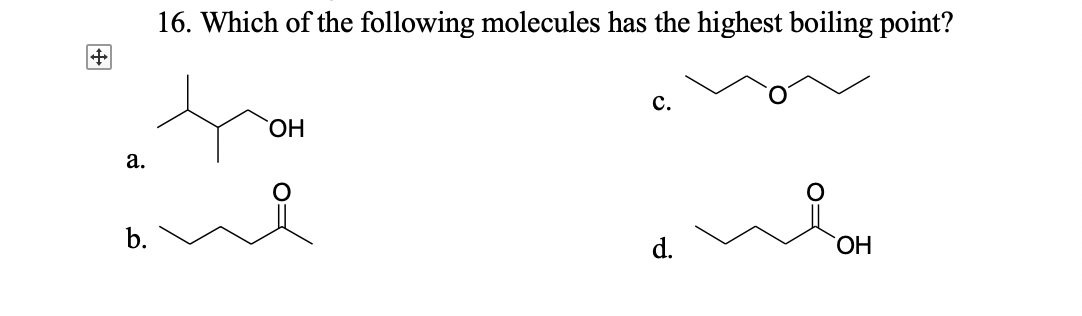 Source: bartleby.com
Source: bartleby.com
Can someone show me how i am supposed to solve these questions step by step though? (a) the higher the boiling point. Acetic acid boiling point is.
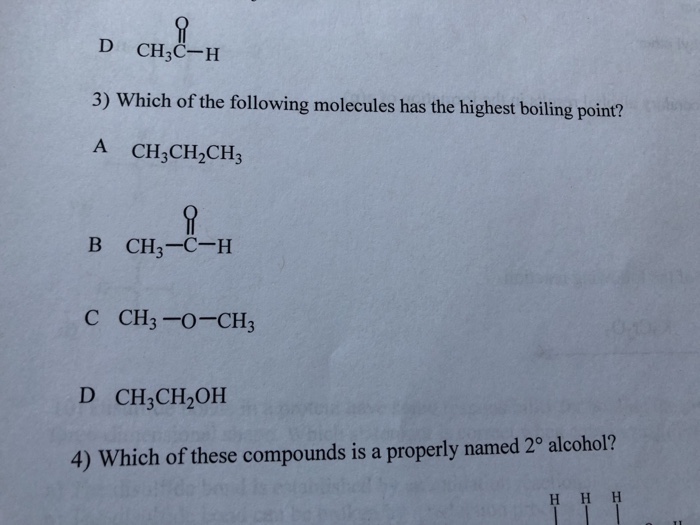
(a) the higher the boiling point. (a) ch4 (b) he (c) hf (d) cl2 3. Which of the following has the highest boiling point?
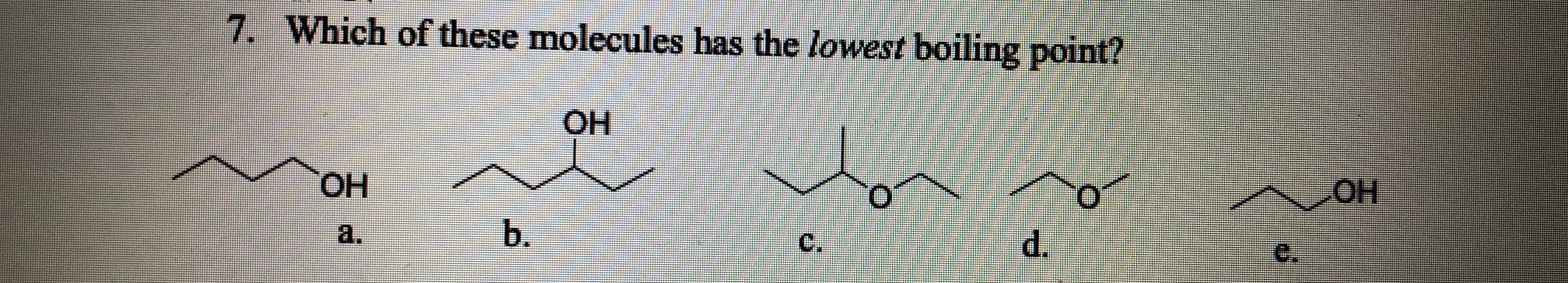 Source: bartleby.com
Source: bartleby.com
What compound has the highest boiling point? In this case, hcl, hbr and hi all have dipoles, but ldf forces appear to be more important in determining the boiling point Dimethyl ether, ch_3och_3, is a polar molecule.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The compound with the highest intermolecular forces will have the highest boiling point. Acetic acid boiling point is. (a) the higher the boiling point.
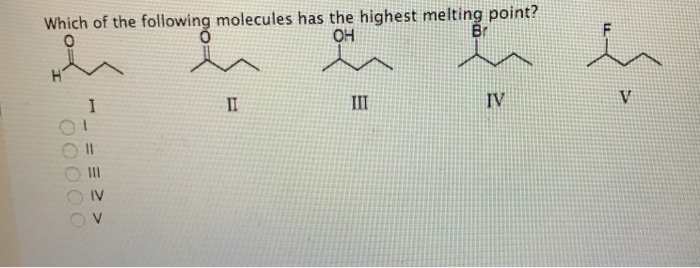
Which of the following has the highest boiling point? Q4 rank the following molecule according to their increasing stability. F2, cl2, br2, i2 which substance has the highest/lowest melting/boiling point, etc.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Methanol has strong hydrogen bonds. In the dotted box write the iupac name of each of the molecules drawn below. (b) the lower the boiling point.
Also Read :





