Species with sp 2 hybridization are plane triangular in shape. Start by determining the lewis structure, then the molecular geometry of the molecules.
Which Of The Following Molecules Has A Trigonal Planar Shape. Predict the shape of the molecule h2co trigonal planar what is the process by which new kinds of orbitals of equal energy are formed from a combination of orbitals of different energies? Skeletal structure skeletal structure final structure final structure shape? Which of the following molecules has a bent structure? Which of the following could be the molecular geometry of the molecule?
 6.3 Molecular Shape | Introductory Chemistry From courses.lumenlearning.com
6.3 Molecular Shape | Introductory Chemistry From courses.lumenlearning.com
Related Post 6.3 Molecular Shape | Introductory Chemistry :
Predict the molecular shape and give the approximate bond angles in the sih4 molecule. Maintaining a positive charge is easier than negative charge and negative charge makes the shape to pyramidal. Electron pairs = (6+4)/2 = 5. There are 3 pairs of electrons around the carbon atom and c− atom in ch 3+.
Trigonal planar molecular geometry is a model which molecule�s shape is triangular and in one plane.
B f 3 molecule is trigonal planar in geometry and is s p 2 hybridised. Phosphorus chloride (pcl 3), aluminium chloride (alcl 3), boron trifluoride (bf 3), aluminium trihydride (alh 3), sulphur (vi) oxide (so 3), etc. Species with sp 2 hybridization are plane triangular in shape. B f 3 molecule is trigonal planar in geometry and is s p 2 hybridised. Molecules adjust their shapes to keep which of the following as far apart as possible? There are 3 pairs of electrons around the carbon atom and c− atom in ch 3+.
 Source: brainly.com
Source: brainly.com
The positive charge does not. Consider an ab 3 molecule in which a and b differ in electronegativity. Bf₃ has a trigonal planar geometry.
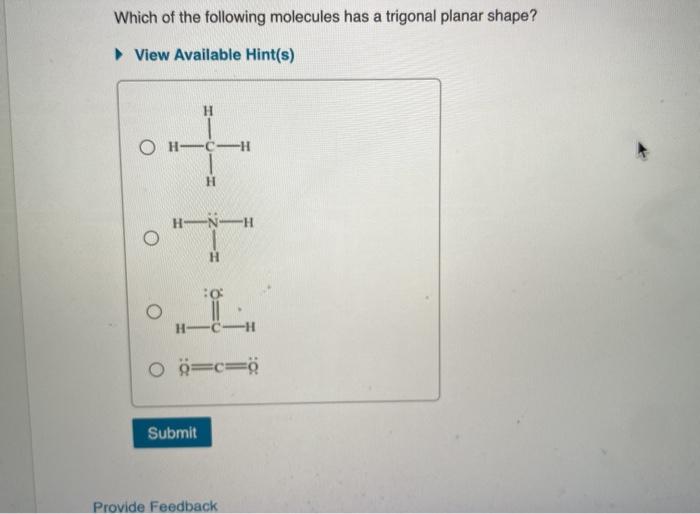
A) the o=s=o bond angle is 180 degrees b) their shape is based on having two lone pairs and two double bond pairs c) their shape is based on then having one lone pair and two bond pairs d)they are trigonal planar Number of valence electrons = 6. Phosphorus chloride (pcl 3), aluminium chloride (alcl 3), boron trifluoride (bf 3), aluminium trihydride (alh 3), sulphur (vi) oxide (so 3), etc.
 Source: chemistry.elmhurst.edu
Source: chemistry.elmhurst.edu
We know that planar structure is the one in which metal atoms and all its ligands are in one plane. Molecules with an trigonal planar electron pair geometries have sp 2 d hybridization at the central atom. Which of the following molecules or ions has a trigonal pyramid shape?
 Source: clutchprep.com
Source: clutchprep.com
Which of the following shapes has unshared pairs of electrons on the central atom? Let�s look at a few chemical compounds that have a trigonal planar geometry. A molecule has a permanent dipole moment if it contains polar bonds and is not a symmetrical shape.
 Source: intl.siyavula.com
Source: intl.siyavula.com
All three are atoms, so this molecule is trigonal planar. The molecule has a trigonal planar electron domain geometry with one lone pair. (the central atom is always first in.
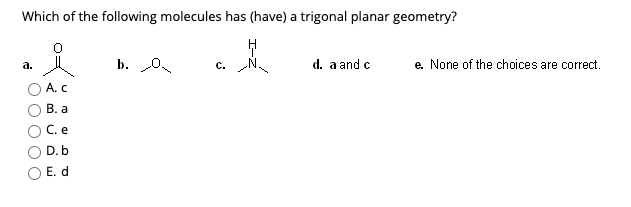 Source: chegg.com
Source: chegg.com
Species with sp 2 hybridization are plane triangular in shape. The geometry of the bf 3 molecule is called trigonal planar the fluorine atoms are positioned at the vertices of an equilateral triangle. So, the answer is d.
 Source: slideplayer.com
Source: slideplayer.com
We know that planar structure is the one in which metal atoms and all its ligands are in one plane. Which of the following molecules has a bent structure? A molecule has a permanent dipole moment if it contains polar bonds and is not a symmetrical shape.
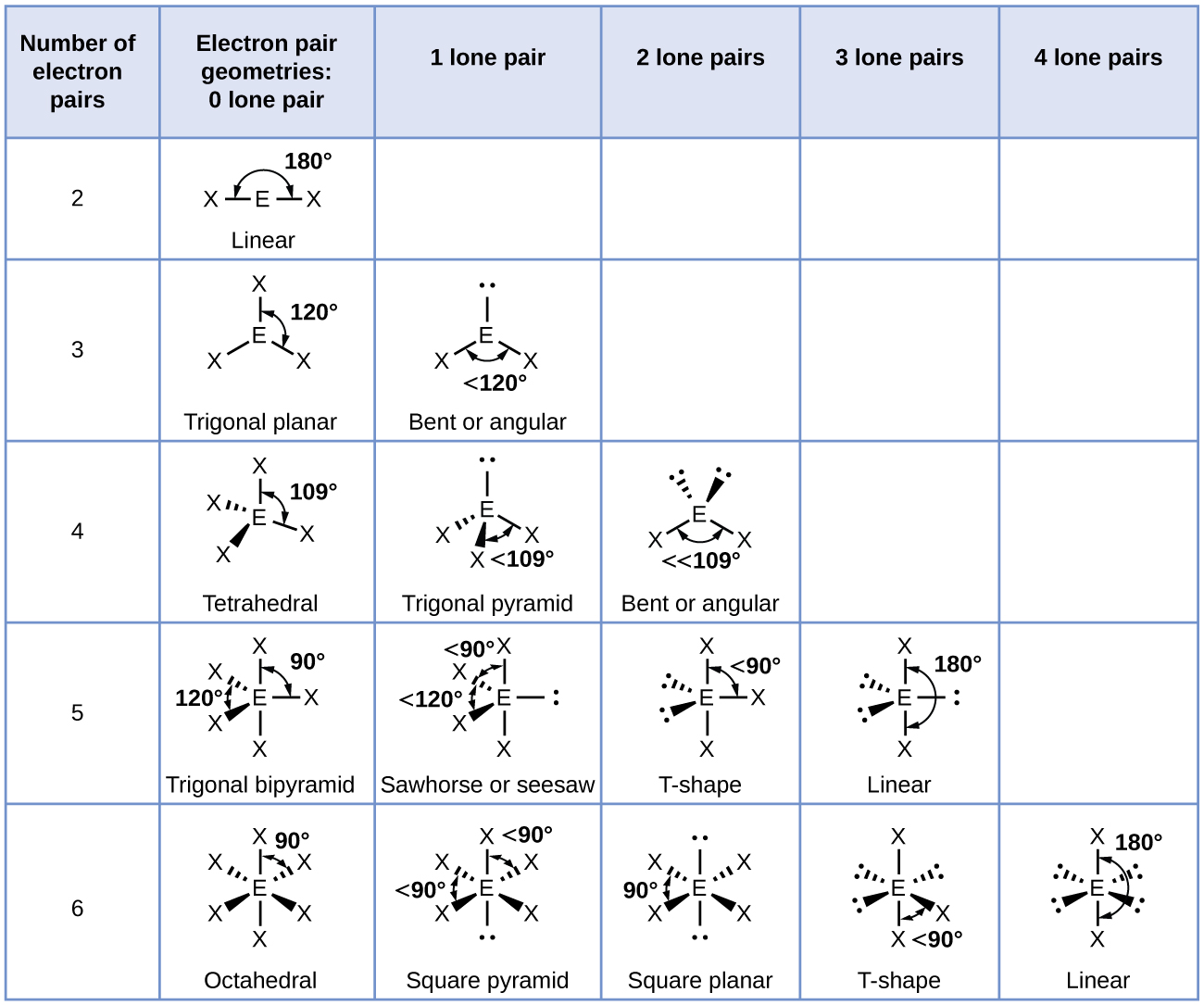 Source: opentextbc.ca
Source: opentextbc.ca
The methyl carbocation $ (c{h_3}^ + ) $ has $ s{p^2} $ hybridisation due to which it has a trigonal planar shape. Phosphorus chloride (pcl 3), aluminium chloride (alcl 3), boron trifluoride (bf 3), aluminium trihydride (alh 3), sulphur (vi) oxide (so 3), etc. Such molecule has three regions of electron density extending out from the central atom and the repulsion will be at minimum when angle between any two is 120°.

Which of the following molecules or ions has a trigonal pyramid shape? The bond angle for a trigonal planar molecule is. The compounds boron hydride (bh 3) and boron trifluoride (bf 3).
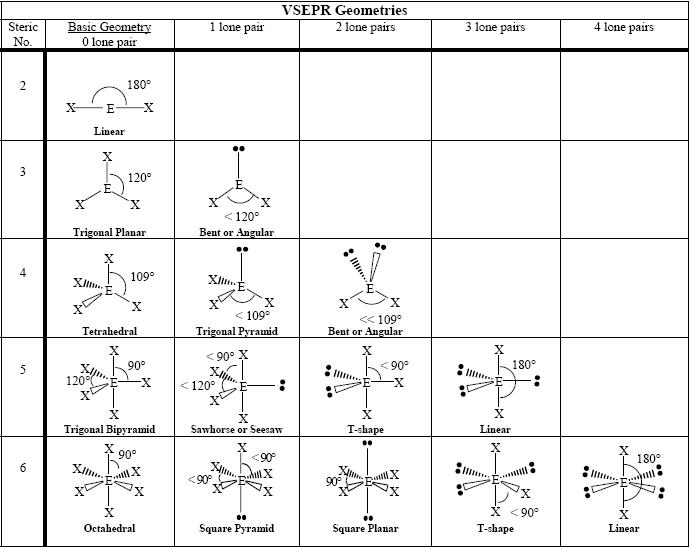 Source: socratic.org
Source: socratic.org
The molecule has a trigonal planar electron domain geometry with one lone pair. A molecule has a permanent dipole moment if it contains polar bonds and is not a symmetrical shape. Species with sp 2 hybridization are plane triangular in shape.
 Source: pinterest.com
Source: pinterest.com
The methyl carbocation $ (c{h_3}^ + ) $ has $ s{p^2} $ hybridisation due to which it has a trigonal planar shape. A+b2 where a and b are two different elements so this type of molecule will always be planar whatever it’s hybridization is that doesn’t matter. Calculate the total number of bond pairs and lone pairs and hence predict the shape of the sf 4 molecule.
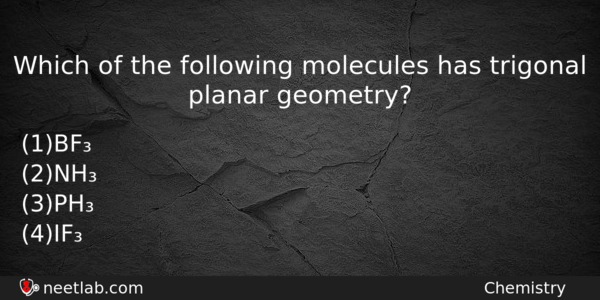 Source: neetlab.com
Source: neetlab.com
Calculate the total number of bond pairs and lone pairs and hence predict the shape of the sf 4 molecule. A molecule has a permanent dipole moment if it contains polar bonds and is not a symmetrical shape. Thus the molecule has a trigonal bipyramidal shape with a t shaped structure.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Shape of the molecule around each one. There are 3 pairs of electrons around the carbon atom and c− atom in ch 3+. Calculate the total number of bond pairs and lone pairs and hence predict the shape of the sf 4 molecule.
 Source: brainly.com
Source: brainly.com
The red triangle represents the outward shape formed by the orbitals, which is triangular (or trigonal ). Number of valence electrons = 6. In water molecules there are two lone pairs on oxygen.
 Source: brainly.com
Source: brainly.com
Which of the following molecules has a bent structure? You are told that the molecule has an overall dipole moment of zero. However, pcl₃ and nh₃ have sp³ hybridization and have a pyramidal shape.
 Source: brainly.com
Source: brainly.com
We know that planar structure is the one in which metal atoms and all its ligands are in one plane. Which of the following could be the molecular geometry of the molecule? Predict the shape of the molecule h2co trigonal planar what is the process by which new kinds of orbitals of equal energy are formed from a combination of orbitals of different energies?
![Solved:question 3 Which One Of The Following Molecules Has & Trigonal Bipyramidal Molecular Geometry? Pf3 [Poa]? [So3]2 - Brfa So3 All Of The Above None Of The Above Solved:question 3 Which One Of The Following Molecules Has & Trigonal Bipyramidal Molecular Geometry? Pf3 [Poa]? [So3]2 - Brfa So3 All Of The Above None Of The Above](https://cdn.numerade.com/ask_images/b2d7abc5357e443eb86ac82373fb65ab.jpg) Source: numerade.com
Source: numerade.com
Consider an ab 3 molecule in which a and b differ in electronegativity. The red triangle represents the outward shape formed by the orbitals, which is triangular (or trigonal ). What kind of geometry does the following molecule have?
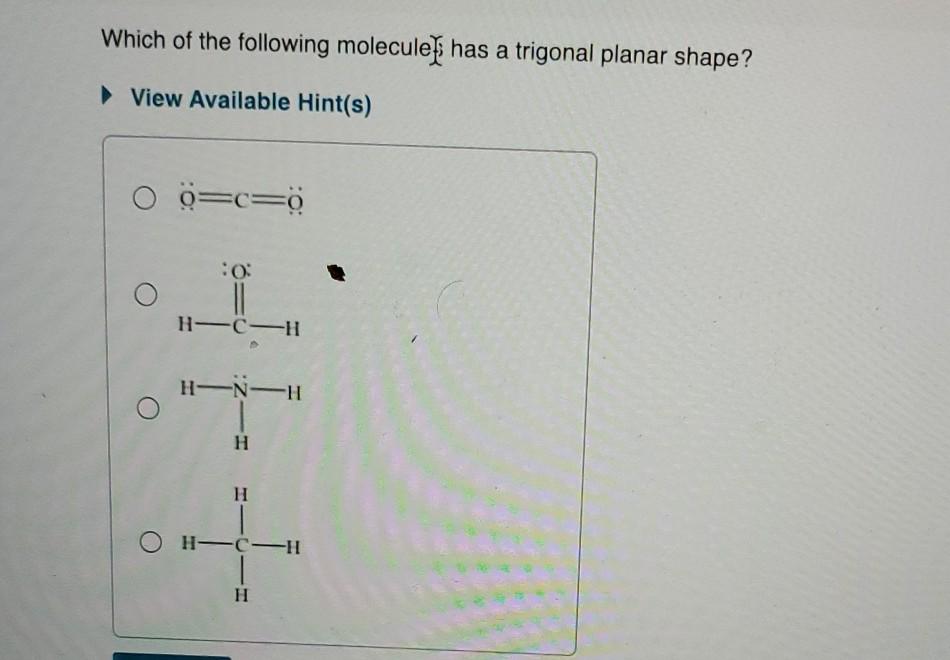
Thus the molecule has a trigonal bipyramidal shape with a t shaped structure. Shape of the molecule around each one. Which of the following could be the molecular geometry of the molecule?
 Source: quizlet.com
Source: quizlet.com
Number of valence electrons = 6. The red triangle represents the outward shape formed by the orbitals, which is triangular (or trigonal ). The lewis structure of each molecule is shown in the attachment.
 Source: studylib.net
Source: studylib.net
Electron pairs = (6+4)/2 = 5. Maintaining a positive charge is easier than negative charge and negative charge makes the shape to pyramidal. Molecules with an trigonal planar electron pair geometries have sp 2 d hybridization at the central atom.
Also Read :





