A) h2o2 b) h2o c) c2h2 d) c4h8. The ratio of atoms in that species is 1:2:1 with respect to carbon, hydrogen, and oxygen.
Which Of The Following Is The Empirical Formula For C4h8. Aniline, consists of c, h, and n. Convert grams c4h8 to moles or moles c4h8 to grams. The number of structural isomers possible for c4h8 is 1 4 2 3 3 5 4 6. B 3 n 3 h 6.
 Solved Of The Following, The Only Empirical Formula Is O | Chegg.com From chegg.com
Solved Of The Following, The Only Empirical Formula Is O | Chegg.com From chegg.com
Related Post Solved Of The Following, The Only Empirical Formula Is O | Chegg.com :
There are a number of types of isomers. 12.01074 + 1.007948 ›› percent composition by element 17) each of the following is a valid molecular formula. Which of the following shows an empirical formula?
What is the molecular formula of each compound?
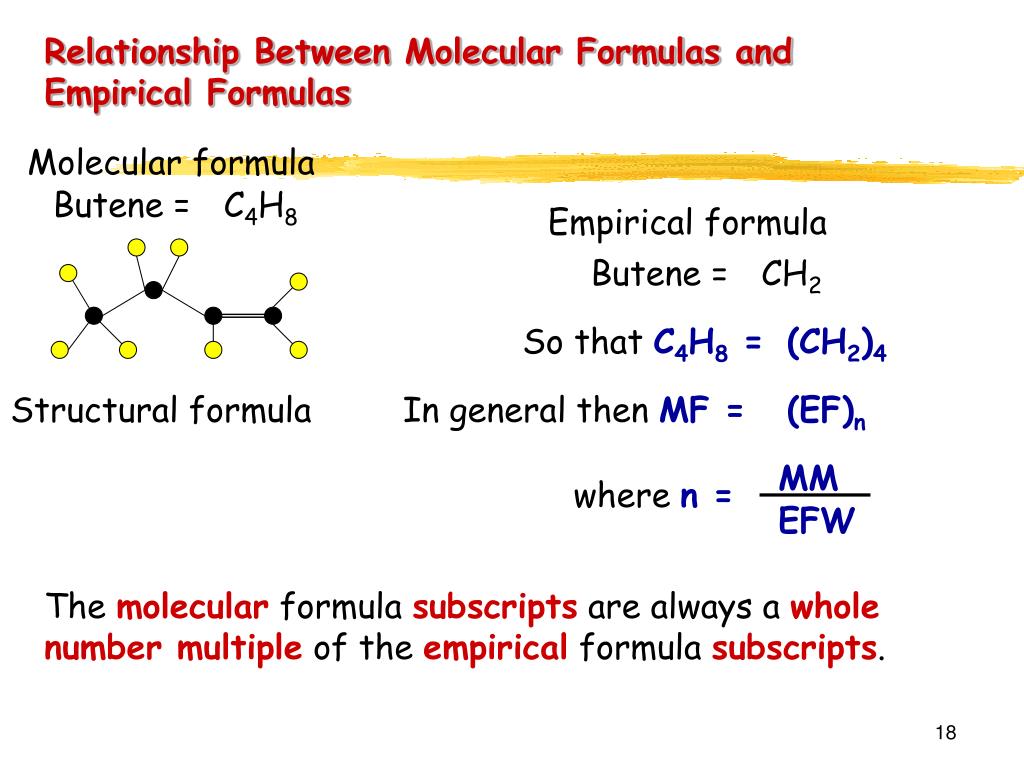
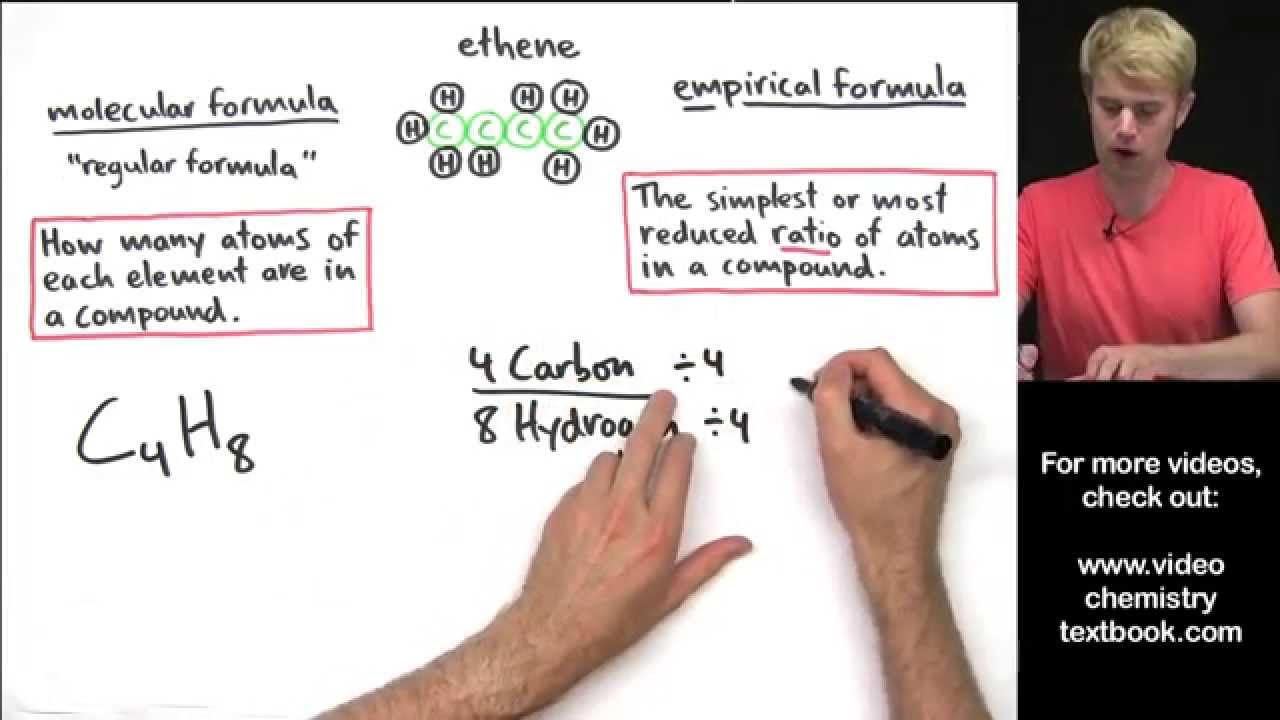
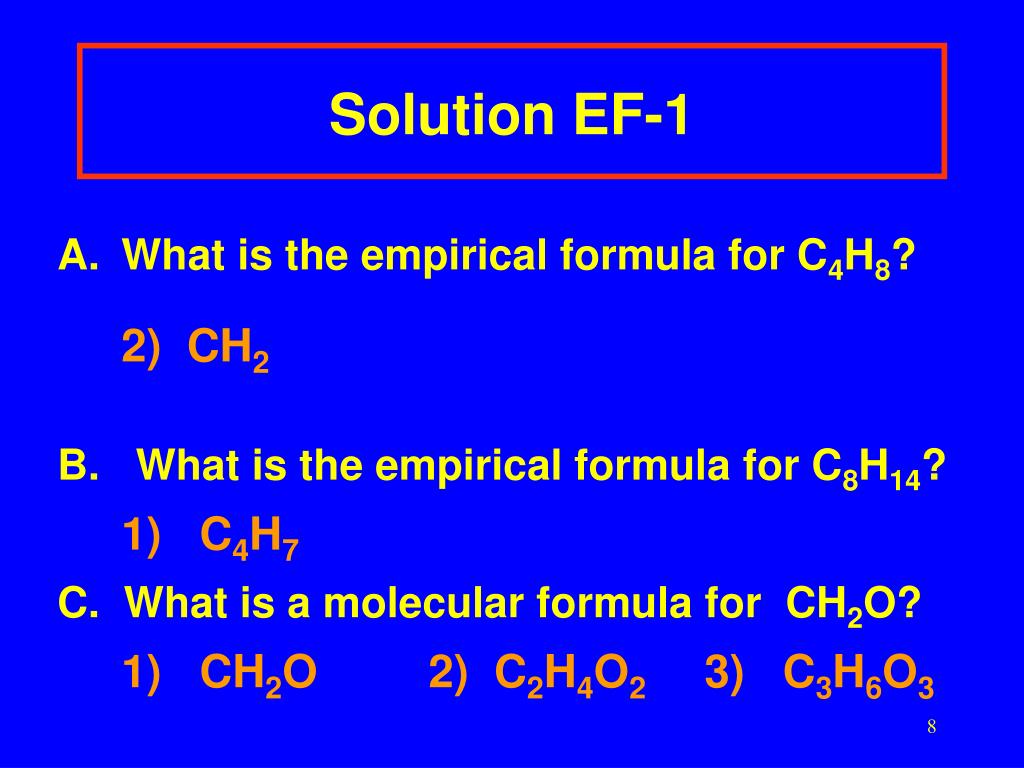
C 4 h 8 ratio: 4:8 ≈ 1:2 c 4 h 8 ÷ 4 = ch 2 empirical formula mass: (a) €€€€write an equation for each of the following steps in the mechanism for the reaction of chloromethane (ch. The empirical formula of pvc is the same as the empirical formula of its monomer. We know that the molecules with the same molecular formula but different molecular geometries are called isomers. What is the molecular formula of each compound?
 Source: slideserve.com
Source: slideserve.com
The ratio of atoms in that species is 1:2:1 with respect to carbon, hydrogen, and oxygen. (2) (total 5 marks) chlorine can be used to make chlorinated alkanes such as dichloromethane. What is the empirical formula and the empirical formula mass for each of the following compounds:
 Source: youtube.com
Source: youtube.com
Which of the following is an empirical formula? In everyday use, the iupac nomenclature is less common than the use of older designations. What is the molecular formula of a compound with the molecular weight of 372.504 amu?
 Source: researchgate.net
Source: researchgate.net
What prefixes should be used for the following hydrocarbon molecules? What is the molecular formula of each compound? 17) each of the following is a valid molecular formula.
 Source: toppr.com
Source: toppr.com
Convert grams c4h8 to moles or moles c4h8 to grams. You’re just simplifying c4h8, 4 can go into c4 1 time (so we just say c) and 4 can go into h8 2 times (h2) An empirical formula shows the ratio of elements present but not the actual number or.
 Source: slideserve.com
Source: slideserve.com
Which of the following is an empirical formula? 1) c2h4 2) ch2 3) ch b. You’re just simplifying c4h8, 4 can go into c4 1 time (so we just say c) and 4 can go into h8 2 times (h2)

If the combustion of 9.71 mg of aniline yields 6.63 mg h2o and 1.46 mb n2, what is the empirical formula? What is the molecular formula of each compound? A)c2n2h8 b)sb4s6 c)be2(cr2o7)2 d) c3h8o
 Source: slideplayer.com
Source: slideplayer.com
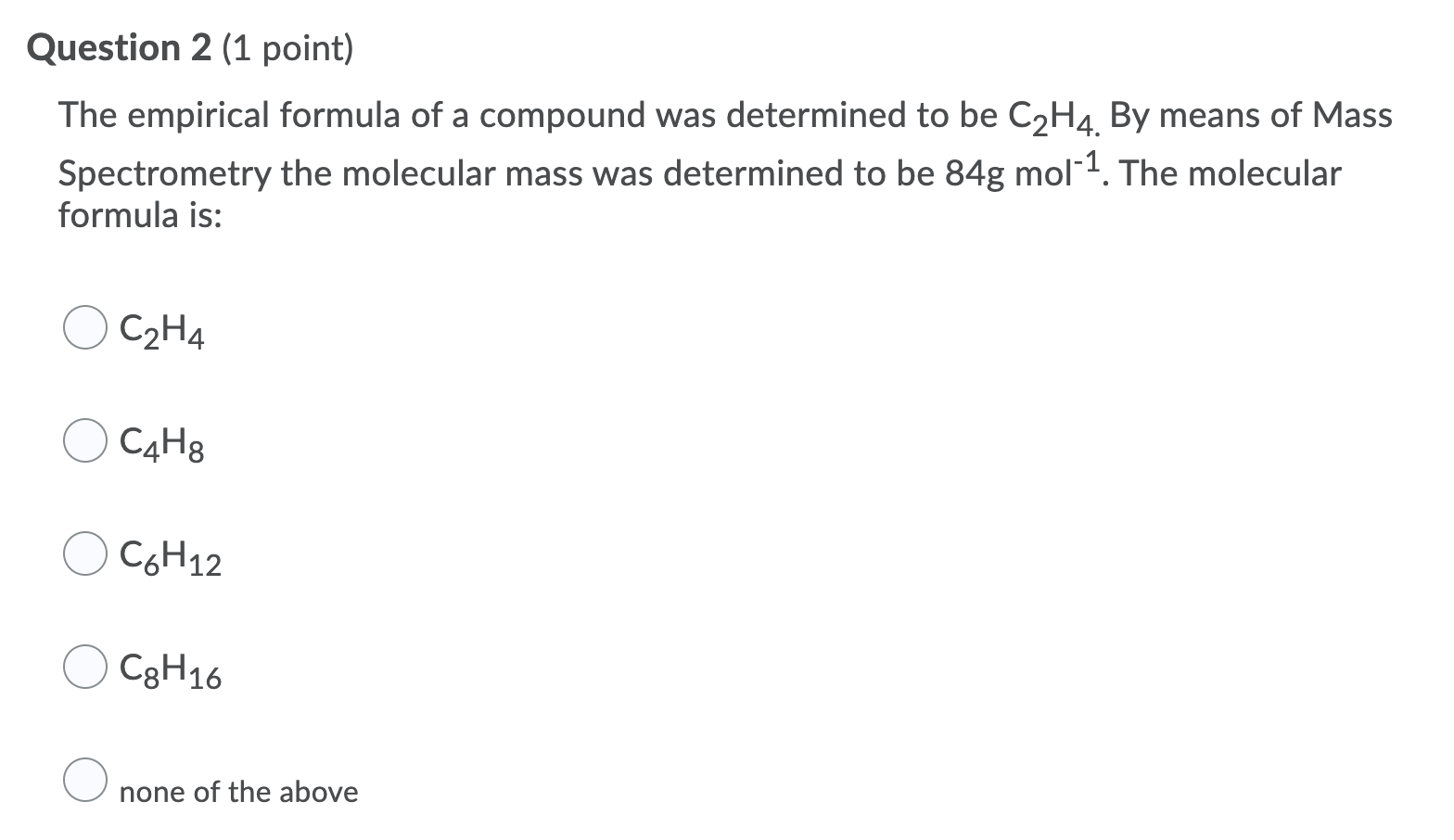
Which of the following is the empirical formula for c4h8? You’re just simplifying c4h8, 4 can go into c4 1 time (so we just say c) and 4 can go into h8 2 times (h2) What is the empirical formula and the empirical formula mass for each of the following compounds:

If the combustion of 9.71 mg of aniline yields 6.63 mg h2o and 1.46 mb n2, what is the empirical formula? Which of the following shows an empirical formula? C 4 h 8 ratio:
 Source: studylib.net
Source: studylib.net
What is the empirical formula and empirical formula mass for each of the following compounds. A) h2o2 b) h2o c) c2h2 d) c4h8. Is c4h8 an empirical formula?
 Source: chegg.com
Source: chegg.com
C = 12.01 g/mol h =. Combustion of such compounds yields co2, h2o, and n2. If the combustion of 9.71 mg of aniline yields 6.63 mg h2o and 1.46 mb n2, what is the empirical formula?

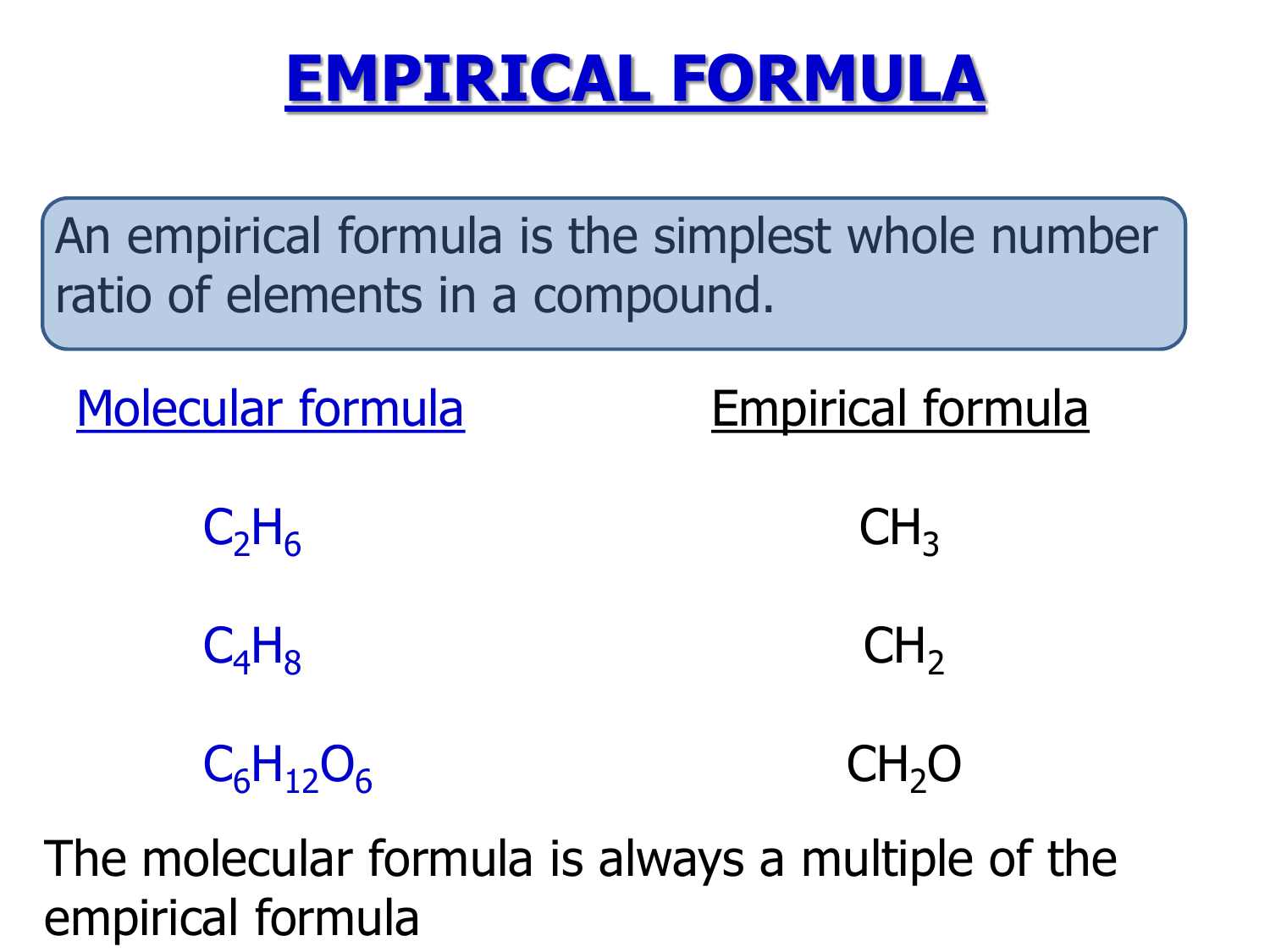
Butene is c4h8, or four times the empirical formula; Which is also an empirical formula? Timberlake lectureplus 3 • an empirical formula represents the simplest whole number ratio of the atoms in a compound.
 Source: slideserve.com
Source: slideserve.com
• the molecular formula is the true or actual ratio of the atoms in a compound. An empirical formula shows the ratio of elements present but not the actual number or. The empirical formula of pvc is the same as the empirical formula of its monomer.
 Source: elevise.co.uk
Source: elevise.co.uk
A)c2n2h8 b)sb4s6 c)be2(cr2o7)2 d) c3h8o A) h2o2 b) h2o c) c2h2 d) c4h8. Timberlake lectureplus 3 • an empirical formula represents the simplest whole number ratio of the atoms in a compound.
 Source: chegg.com
Source: chegg.com
Chemistry q&a library write the empirical formula for the following compounds c4h8 h2o2 co2 Which of the following shows an empirical formula? What is the empirical formula and the empirical formula mass for each of the following compounds:
 Source: quizlet.com
Source: quizlet.com
Which of the following is an empirical formula? Mass of co2 given in the problem is 8.5900g , convert the mass to moles by dividing mass in grams with the molar mass of co2. 17) each of the following is a valid molecular formula.
 Source: socratic.org
Source: socratic.org
Which of the following is an empirical formula? Which of the following is an empirical formula? A)c2n2h8 b)sb4s6 c)be2(cr2o7)2 d) c3h8o
 Source: slideplayer.com
Source: slideplayer.com
May 4, 2021 by admin. An empirical formula shows the ratio of elements present but not the actual number or. Which of the following shows an empirical formula?
 Source: slideplayer.com
Source: slideplayer.com
C 4 h 8 ratio: Butenes are unsaturated olefinic hydrocarbons, c4h8, mw 56.1080, existing in four isomers: What is the empirical formula and the empirical formula mass for each of the following compounds:

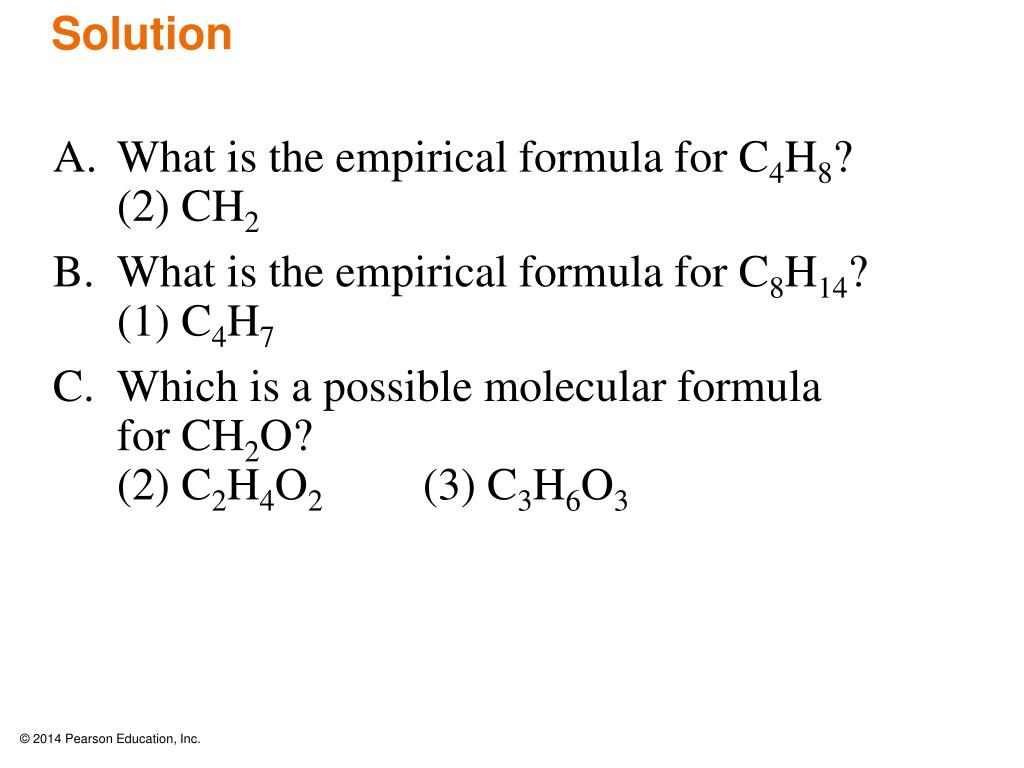
- c2h4 2) ch2 3) ch b. Here (en)n = m f = (ch 2o) × 3 = c3h 6o3 as required. What is the empirical formula and empirical formula mass for each of the following compounds?
 Source: researchgate.net
Source: researchgate.net
(a) €€€€write an equation for each of the following steps in the mechanism for the reaction of chloromethane (ch. Is c4h8 an empirical formula? (2) (total 5 marks) chlorine can be used to make chlorinated alkanes such as dichloromethane.
Also Read :