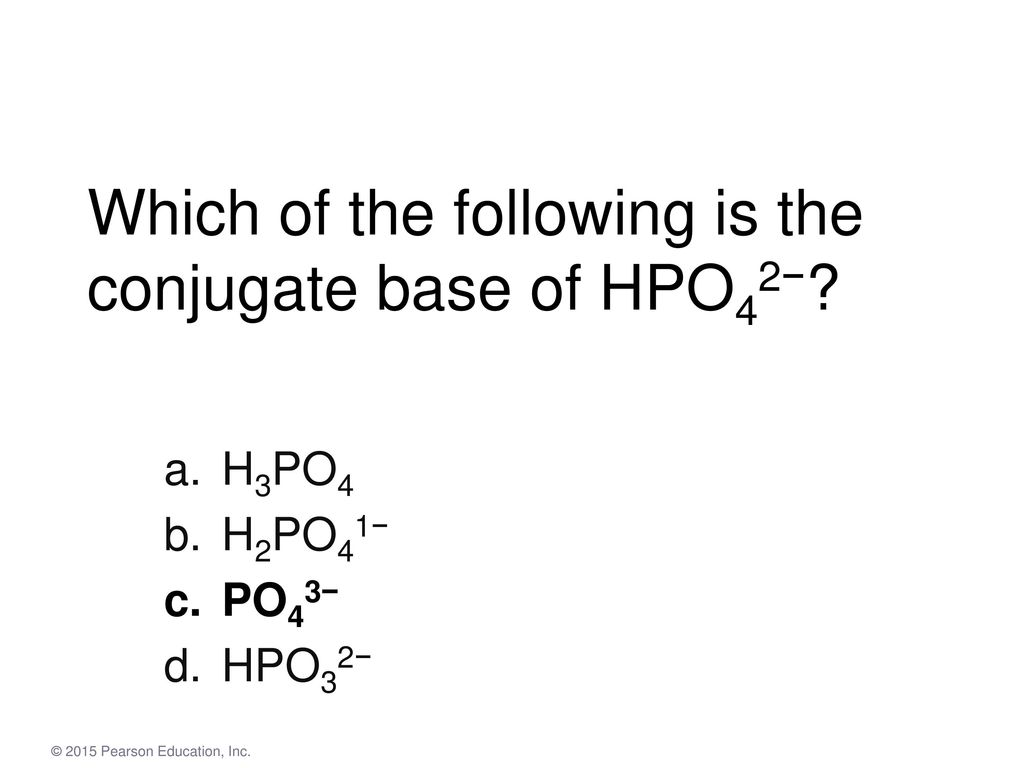
Which of the following is conjugate base of [c 2. A) the stronger the conjugate acid.
Which Of The Following Is The Conjugate Base Of H3po4. What is the conjugate base of h3po4? Identify the conjugate base of the following acids [co (nh3)5 (oh2)]3. You can tell that h3po4 (phosphoric acid) is the acid because in. H2o gained a proton to form h3o^+;
![Identify The Conjugate Base Of The Following Acids [Co(Nh3)5(Oh2)]3 - Nationpulse Identify The Conjugate Base Of The Following Acids [Co(Nh3)5(Oh2)]3 - Nationpulse](https://media.cheggcdn.com/media%2F4e8%2F4e84a3ff-bf97-43cf-be54-37d0e5e64dc8%2Fimage)
Related Post Identify The Conjugate Base Of The Following Acids [Co(Nh3)5(Oh2)]3 - Nationpulse :
C) the weaker the conjugate base. 18 related question answers found what does conjugate base mean? Question 3 identify the conjugate base of h3po4. This conjugate base is called…
Provide the formula of the conjugate base for each of the following acids:
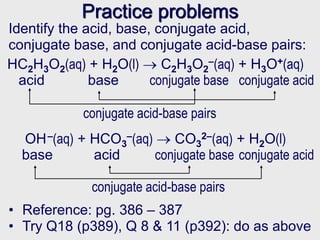
The partners in an acid / base conjugate pair differ from one other by a single hydrogen ion. Provide the formula of the conjugate base for each of the following acids: It is true that a) h3po4 is the conjugate acid. To identify what the conjugate species are, the backward reaction is considered. The partners in an acid / base conjugate pair differ from one other by a single hydrogen ion. The species that is formed is the acids conjugate base.
 Source: chegg.com
Source: chegg.com
Write the chemical formula of each of the following: A) the stronger the conjugate acid. The compound ion hpo4 is called hydrogen phosphate.
 Source: slideplayer.com
Source: slideplayer.com
Nh4+ is the conjugate acid to the base nh3, because nh3 gained a hydrogen ion to form nh4+.the conjugate base of an acid is formed when the acid donates a proton. D) more than one correct response e) no correct response D) the conjugate base of a very weak acid is stronger than the conjugate base of a strong acid.
 Source: youtube.com
Source: youtube.com
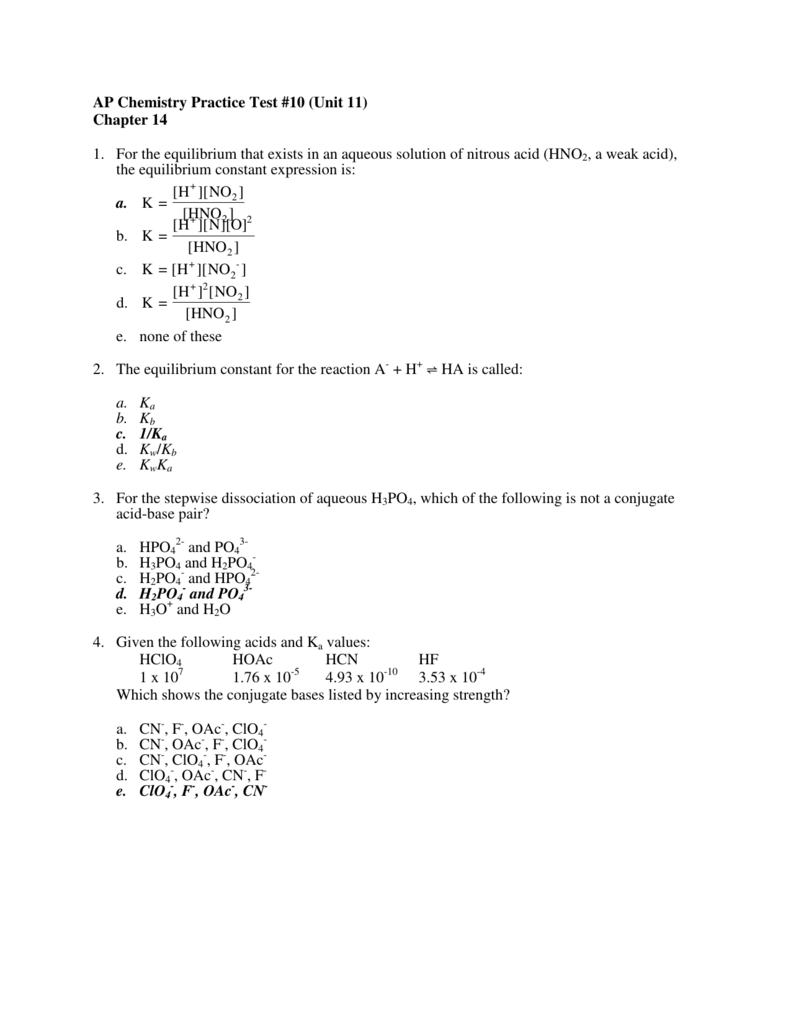
- the stronger the acid, then which of the following is true? A) the stronger the conjugate acid. Question 3 identify the conjugate base of h3po4.
 Source: ask.learncbse.in
Source: ask.learncbse.in
So3 (g) + so2 (g) +1/2 02 (g) kp= 1.85 at 1000k 0.204 4.2 0.0203 16.8. 18 related question answers found what does conjugate base mean? In classical term a base is defined as a compound which reacts with an acid to form salt and water as depicted by the following equation.
![Identify The Conjugate Base Of The Following Acids [Co(Nh3)5(Oh2)]3 - Nationpulse Identify The Conjugate Base Of The Following Acids [Co(Nh3)5(Oh2)]3 - Nationpulse](https://media.cheggcdn.com/media%2F4e8%2F4e84a3ff-bf97-43cf-be54-37d0e5e64dc8%2Fimage)
C) the weaker the conjugate base. Nh4+ is the conjugate acid to the base nh3, because nh3 gained a hydrogen ion to form nh4+.the conjugate base of an acid is formed when the acid donates a proton. H2o gained a proton to form h3o^+;
 Source: slideshare.net
Source: slideshare.net
Therefore it is the conjugate base. The partners in an acid / base conjugate pair differ from one other by a single hydrogen ion. Question 3 identify the conjugate base of h3po4.
 Source: chegg.com
Source: chegg.com
So3 (g) + so2 (g) +1/2 02 (g) kp= 1.85 at 1000k 0.204 4.2 0.0203 16.8. 18 related question answers found what does conjugate base mean? Nh4+ is the conjugate acid to the base nh3, because nh3 gained a hydrogen ion to form nh4+.the conjugate base of an acid is formed when the acid donates a proton.
 Source: studylib.net
Source: studylib.net
Provide the formula of the conjugate base for each of the following acids: The species that is formed is the acids conjugate base. In classical term a base is defined as a compound which reacts with an acid to form salt and water as depicted by the following equation.

This conjugate base is called… 2) the stronger the acid, then which of the following is true? Therefore, h2o is the base and h3o^+ is its conjugate acid.
 Source: toppr.com
Source: toppr.com
18 related question answers found. Conjugate base definition when an acid dissociates into its ions in water, it loses a hydrogen ion. C) the weaker the conjugate base.
 Source: clutchprep.com
Source: clutchprep.com
- what is the conjugate base of h2po4? H2o gained a proton to form h3o^+; Question 3 identify the conjugate base of h3po4.
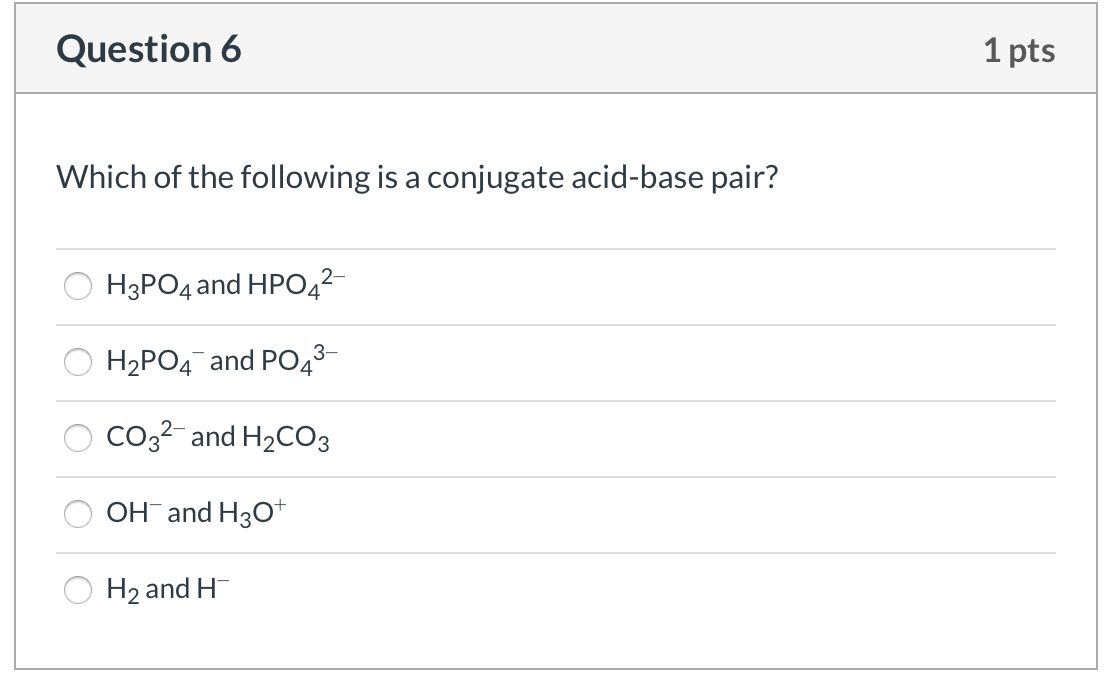 Source: chegg.com
Source: chegg.com
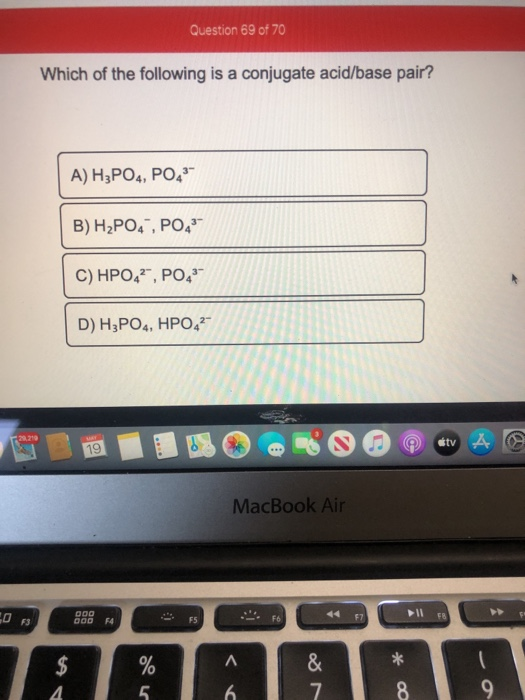
Which of the following is a conjugate acid/ base pair? C) the weaker the conjugate base. What is the conjugate base of h3po4?
![Solved] 23) Which Of The Following Is Not A Conjugate Acid - Conjugate Base Pair (In That Order)? | Course Hero](https://www.coursehero.com/qa/attachment/7468831/ “Solved] 23) Which Of The Following Is Not A Conjugate Acid - Conjugate Base Pair (In That Order)? | Course Hero”) Source: coursehero.com
Write the chemical formula of each of the following: Question 3 identify the conjugate base of h3po4. C) the weaker the conjugate base.

The compound ion hpo4 is called hydrogen phosphate. Therefore it is the conjugate base. The partners in an acid / base conjugate pair differ from one other by a single hydrogen ion.
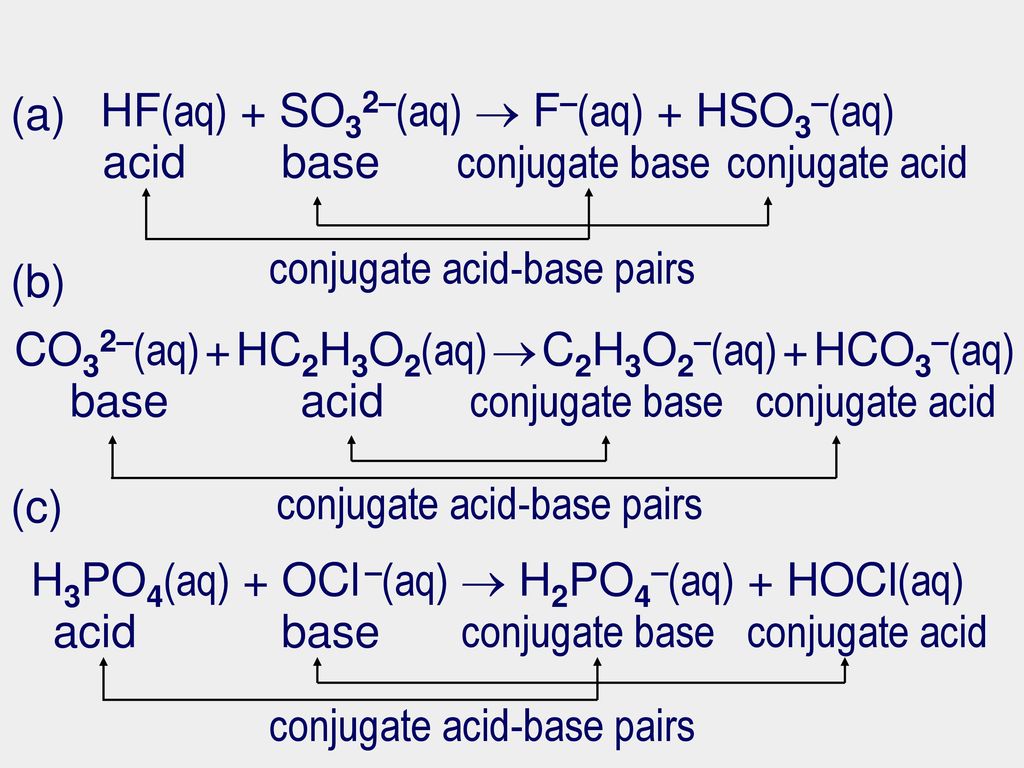 Source: slideplayer.com
Source: slideplayer.com
H2o gained a proton to form h3o^+; The partners in an acid / base conjugate pair differ from one other by a single hydrogen ion. In classical term a base is defined as a compound which reacts with an acid to form salt and water as depicted by the following equation.
 Source: studylib.net
Source: studylib.net
You can tell that h3po4 (phosphoric acid) is the acid because in. This conjugate base is called… In classical term a base is defined as a compound which reacts with an acid to form salt and water as depicted by the following equation.
 Source: clutchprep.com
Source: clutchprep.com
C) the weaker the conjugate base. − is conjugate base of h 3. 18 related question answers found what does conjugate base mean?

Which of the following statements is true? 2) the stronger the acid, then which of the following is true? 18 related question answers found what does conjugate base mean?
 Source: oneclass.com
Source: oneclass.com
C) the weaker the conjugate base. Question 3 identify the conjugate base of h3po4. − is conjugate base of h 3.
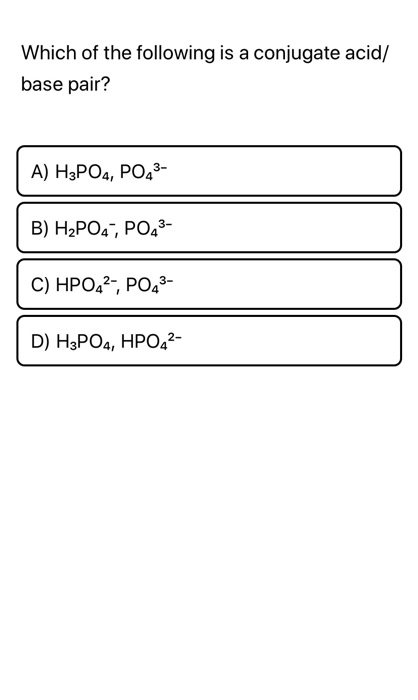 Source: chegg.com
Source: chegg.com
A) the stronger the conjugate acid. H2o gained a proton to form h3o^+; 18 related question answers found.
Also Read :





