5when ethyl 4 aminobenzoate was added into 1. What, if anything, do their atoms have in common.
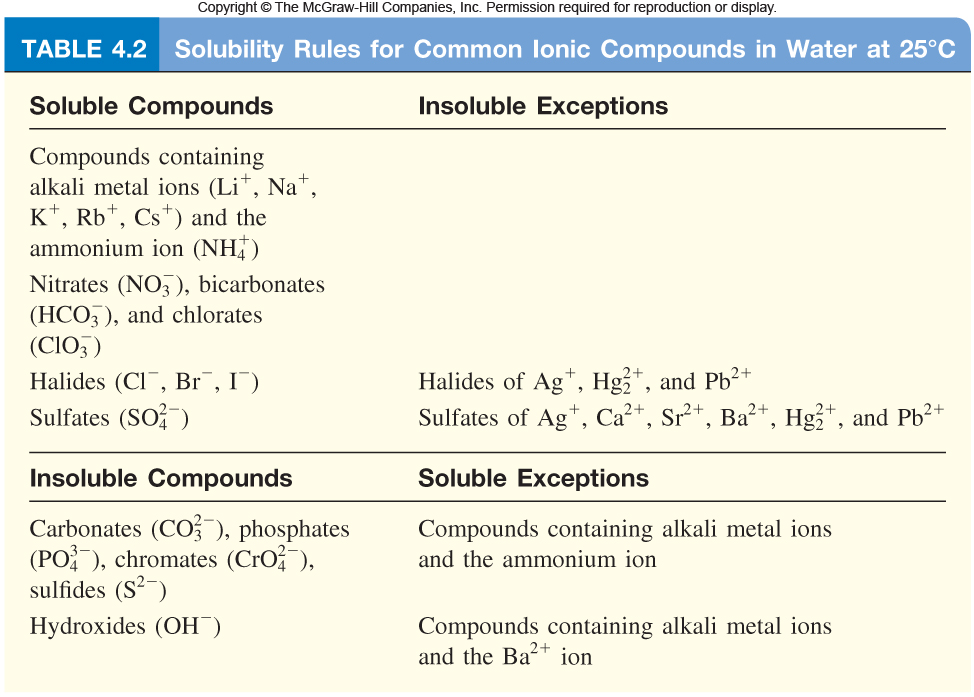
Which Of The Following Is Insoluble In Water. Calcium carbonate is insoluble in water because the electrostatic bonds between the carbonate anion and the calcium ion are too strong to be overcome by solvation by water molecules. In the modem periodic table, which are the metals among the first ten elements. Which one of the following compounds is insoluble in water? Which of the following compounds is insoluble in water?
 Solved Question S Which Of The Following Is Insoluble In | Chegg.com From chegg.com
Solved Question S Which Of The Following Is Insoluble In | Chegg.com From chegg.com
Related Post Solved Question S Which Of The Following Is Insoluble In | Chegg.com :
All of the above which of the following. Calcium carbonate is insoluble in water because the electrostatic bonds between the carbonate anion and the calcium ion are too strong to be overcome by solvation by water molecules. Classify each of the following as a strong electrolyte or non electrolyte.mgbr2, c12h22o11, na2co3, koh q. Which of the following offers potential customers the opportunity to try a product or service before they make a buying decision?
I) all sulfides are insoluble except those of ammonium, sodium, calcium, potassium, magnesium, barium and strontium.
Which of the following offers potential customers the opportunity to try a product or service before they make a buying decision? Here are some things that are mostly insoluble in water: The cookie is used to store the user consent for the cookies in the category analytics. #2 nac2h302 srso, bas aipoa select one: Likewise sand is insoluble in water since water is not strong enough to dissolve sand. C) the primary nitrogenous waste product of humans.
 Source: slideplayer.com
Source: slideplayer.com
Oil does not mix with water because it is nonpolar and hence, does not interact well with the water molecules and floats at the top of the water. Practice exercise 1 which of the following compounds is insoluble in water? Lithium, sodium, potassium are all metals that react with water to.

Here are some things that are mostly insoluble in water: Sodium chloride (nacl) it is the chemical name for salt. Potassium sulfate, or k_2so_4, si soluble because all sulfates are soluble with the exception of those formed with silver, calcium, barium, mercury, lead, and strontium ions, finally, copper (ii) hydroxyde, or cu(oh)_2, is insoluble because all hydroxides are insoluble with the exception of those formed with alkali metal ions and barium.

Get why is ethyl 4 aminobenzoate insoluble in water from 6 different pptx ethyl 4 aminobenzoate was found to be insoluble in water and 10 m naoh but upon addition of 60 m naoh to this solution ethyl 4 aminobenzoate became insoluble. Which one of the following compounds is insoluble in water? It is a) insoluble in water.
 Source: ask.learncbse.in
Source: ask.learncbse.in
Matter can be soluble or insoluble in water. Which of the following offers potential customers the opportunity to try a product or service before they make a buying decision? Solved 1 which one of the following is characteristic of chegg.

Pb2+(aq) to produce an insoluble compound. All ionic compounds are soluble in water to some extent, but the degree of solubility varies. One may also ask, what type of compounds are soluble in water?
This is an exception as halide salt of most of the metal are soluble in water. ((po4)2 is usually insoluble unless paired with li, na, k, or nh4 in water) identify the solid product formed, if any, from the reaction of (nh4)2co3 and mg(no3)2. Predict whether the following compounds are soluble or insoluble in water:nh4br, cubr2, agl, pb(no3)2, cuco3, caso4
 Source: chegg.com
Source: chegg.com
According to solubility rules, which of the following compounds is insoluble in water? Which of the following compounds is insoluble in water? Calculate the volume of koh required to reach the equivalence point.
 Source: bioprofe.com
Source: bioprofe.com
Predict whether the following compounds are soluble or insoluble in water:nh4br, cubr2, agl, pb(no3)2, cuco3, caso4 Calculate the volume of koh required to reach the equivalence point. The halide salts of silver and lead are also insoluble in water.
 Source: science-sparks.com
Source: science-sparks.com
Classify each of the following as a strong electrolyte or non electrolyte.mgbr2, c12h22o11, na2co3, koh q. Calcium carbonate is insoluble in water because the electrostatic bonds between the carbonate anion and the calcium ion are too strong to be overcome by solvation by water molecules. All of the above which of the following.

(a) (nh4)2s, (b) caco3, (c) naoh, (d) ag2so4 (e) pb(ch3coo)2 (a) (nh4)2s, (b) caco3, (c) naoh, (d) ag2so4 (e) pb(ch3coo)2 Potassium sulfate, or k_2so_4, si soluble because all sulfates are soluble with the exception of those formed with silver, calcium, barium, mercury, lead, and strontium ions, finally, copper (ii) hydroxyde, or cu(oh)_2, is insoluble because all hydroxides are insoluble with the exception of those formed with alkali metal ions and barium. 9) which of the following is true of urea?
 Source: chegg.com
Source: chegg.com
A.cal 2 b.na so 2 4 c.agf d.ai(oh) 3 2 see answers advertisement advertisement histrionicus histrionicus answer: Which of the following is a key difference between sweepstakes and contests? 9) which of the following is true of urea?

How many of the following salts are expected to be insoluble in water? It is a) insoluble in water. Lithium, sodium, potassium are all metals that react with water to.
 Source: ask.learncbse.in
Source: ask.learncbse.in
Likewise sand is insoluble in water since water is not strong enough to dissolve sand. A volume of 40.0 ml of a 0.410 m hno3 solution is titrated with 0.110 m koh. Classify each of the following as a strong electrolyte or non electrolyte.mgbr2, c12h22o11, na2co3, koh q.
 Source: toppr.com
Source: toppr.com
Therefore, and are insoluble in water. 5when ethyl 4 aminobenzoate was added into 1. The cookie is used to store the user consent for the cookies in the category analytics.
 Source: kolekcijablogs.blogspot.com
Source: kolekcijablogs.blogspot.com
Helium is an unreactive gas and neon is a gas of extremely low reactivity. Calcium carbonate is insoluble in water because the electrostatic bonds between the carbonate anion and the calcium ion are too strong to be overcome by solvation by water molecules. Therefore, and are insoluble in water.
 Source: chegg.com
Source: chegg.com
Likewise sand is insoluble in water since water is not strong enough to dissolve sand. According to the solubility rule, the sulfate salt of lead and barium are insoluble in water and hence which has sulfate ion is insoluble in water. What, if anything, do their atoms have in common.
 Source: socratic.org
Source: socratic.org
C) the primary nitrogenous waste product of humans. Sodium sulfide ammonium sulfate barium nitrate potassium phosphate (a) (nh4)2s, (b) caco3, (c) naoh, (d) ag2so4 (e) pb(ch3coo)2 (a) (nh4)2s, (b) caco3, (c) naoh, (d) ag2so4 (e) pb(ch3coo)2

All of these are slightly hydrolyzed in water. The halide salts of silver and lead are also insoluble in water. Get why is ethyl 4 aminobenzoate insoluble in water from 6 different pptx ethyl 4 aminobenzoate was found to be insoluble in water and 10 m naoh but upon addition of 60 m naoh to this solution ethyl 4 aminobenzoate became insoluble.
 Source: chegg.com
Source: chegg.com
Ca cl, how many of the following compounds are insoluble in water? Classify each of the following as a strong electrolyte or non electrolyte.mgbr2, c12h22o11, na2co3, koh q. 9) which of the following is true of urea?
 Source: chegg.com
Source: chegg.com
Which of the following compounds is insoluble in water? Oil does not mix with water because it is nonpolar and hence, does not interact well with the water molecules and floats at the top of the water. Sodium is an electrolyte that regulates the amount of water in your body.
Also Read :