Which of the following is considered a strong electrolyte? Weak electrolytes only partially break into ions in water.
Which Of The Following Is Considered A Strong Electrolyte. Nh4no3 is a salt and… Which of the following is considered a strong electrolyte? Baso_4, agno_3, hbr, hno_2 and hc_2h_2o_2 6. Of the following , the strong electrolyte would be nh4no3.
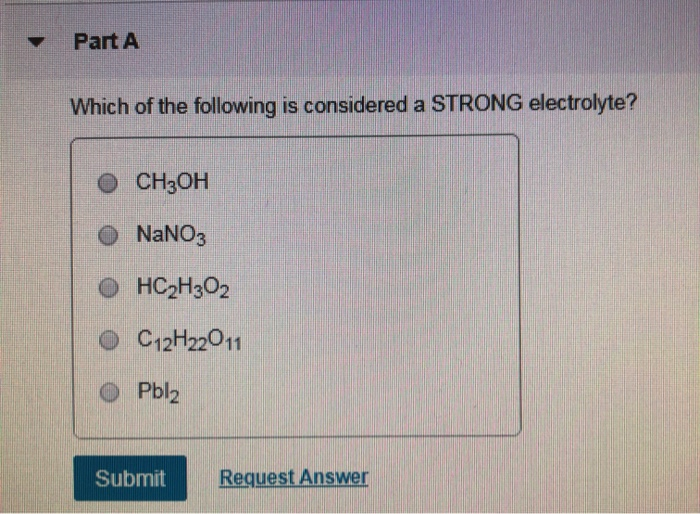 Solved Part A Which Of The Following Is Considered A Strong | Chegg.com From chegg.com
Solved Part A Which Of The Following Is Considered A Strong | Chegg.com From chegg.com
Related Post Solved Part A Which Of The Following Is Considered A Strong | Chegg.com :
C12h22o11 hc2h3o2 pbi2 nh4no3 ch3sh Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Common strong electrolytes are strong acids, strong bases, and ionic salts. Which of the following is considered a strong electrolyte?
These are completely dissociate into ions in aqueous s.
Strong electrolyte definition and examples. thoughtco, aug. A strong electrolyte is a solution/solute that completely, or almost completely, ionizes or dissociates in a solution. Hope this answers the question. N a c l (strong electrolyte) salts are strong electrolytes. Strong electrolyte definition and examples. thoughtco, aug. A) nh 4 no 3 b) c 12 h 22 o 11 c) pbbr 2 d) hc 2 h 3 o 2 e) ch 3 sh
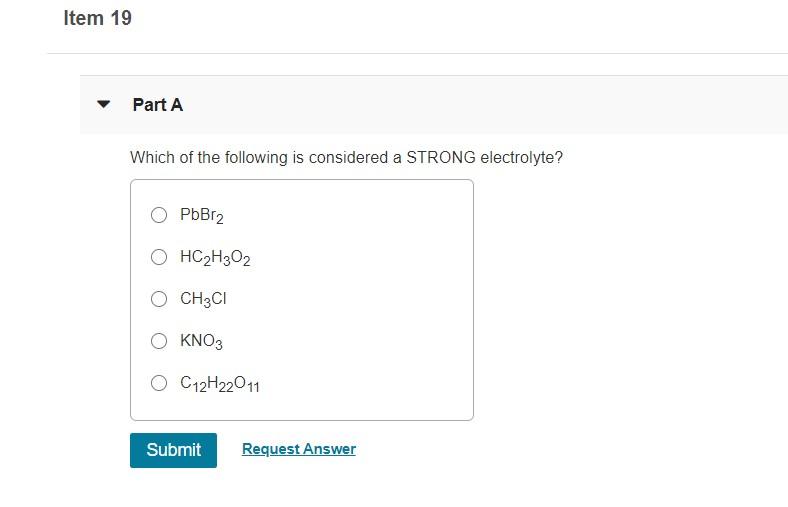
I believe the correct answer from the choices listed above is the fifth option. Which of the following is considered a strong electrolyte? N h 3 (weak electrolyte) b.
 Source: chegg.com
Source: chegg.com
For example, sweating due to heavy exertion can cause electrolyte levels to become low. Most acids are weak electrolytes. I believe the correct answer from the choices listed above is the fifth option.
 Source: clutchprep.com
Source: clutchprep.com
Nh4no3 is a salt and… Most acids are weak electrolytes. Ethanol, c2h6o, nitrogen gas, n2, and water, h2o (i think) are covalently bonded, which rules them out.
 Source: chegg.com
Source: chegg.com
C h 3 c o o h (weak electrolyte) d. So, there are few ions to conduct electricity and the bulb glows dimly. C12h22o11 hc2h3o2 pbi2 nh4no3 ch3sh
 Source: clutchprep.com
Source: clutchprep.com
A) strong electrolyte, weak acid b) weak. Hcl (hydrochloric acid), h 2 so 4 (sulfuric acid), naoh ( sodium hydroxide) and koh (potassium hydroxide) are all strong electrolytes. Sodium, potassium, and chloride are the significant electrolytes along with magnesium, calcium, phosphate, and.
 Source: chegg.com
Source: chegg.com
1which of the following compounds is a strong electrolyte? C h 3 c o o n a (strong electrolyte) e. N h 3 (weak electrolyte) b.
 Source: 10endrathukulla.com
Source: 10endrathukulla.com
Salts much have high solubility in the solvent to act as strong electrolytes. 1which of the following compounds is a strong electrolyte? I believe the correct answer from the choices listed above is the fifth option.

N a c l (strong electrolyte) salts are strong electrolytes. C h 3 c o o h (weak electrolyte) d. Which of the following is considered a strong electrolyte?
 Source: youtube.com
Source: youtube.com
C12h22o11 hc2h3o2 pbi2 nh4no3 ch3sh I.e., there is virtually no undissociated form of the compound in solution. Strong acids, strong bases and soluble ionic salts that are not weak acids or weak bases are strong electrolytes.
 Source: numerade.com
Source: numerade.com
Which of the following is considered a strong electrolyte? N a c l (strong electrolyte) salts are strong electrolytes. A) strong electrolyte, weak acid b) weak.
 Source: sciencenotes.org
Source: sciencenotes.org
N h 3 (weak electrolyte) b. N h 3 (weak electrolyte) b. Nh4no3 is a salt and…
 Source: chegg.com
Source: chegg.com
N h 3 (weak electrolyte) b. Of the following , the strong electrolyte would be nh4no3. These are completely dissociate into ions in aqueous s.
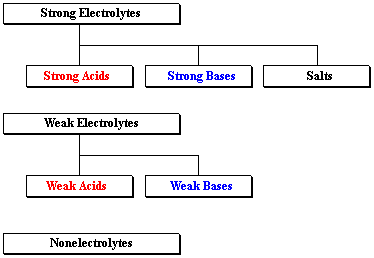 Source: stolaf.edu
Source: stolaf.edu
Nh4no3 is a salt and… Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells, generating and conducting action potentials in the nerves and muscles. Use the guidelines to check your answer:
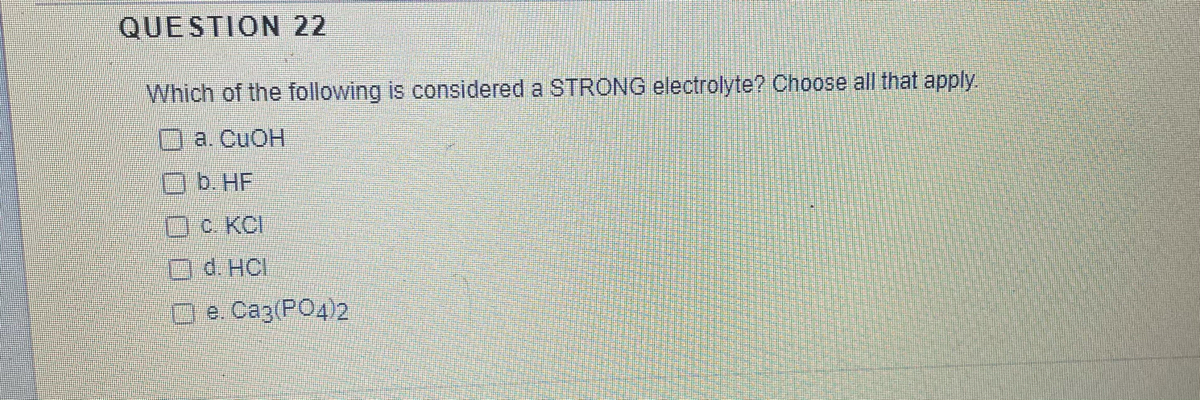 Source: bartleby.com
Source: bartleby.com
Nh4no3 is a salt and… Most acids are weak electrolytes. Nh4no3 is a salt and completely dissociates in aqueous solution.
 Source: numerade.com
Source: numerade.com
C h 3 c o o h (weak electrolyte) d. 2which of the following compounds is a strong electrolyte? Hcl (hydrochloric acid), h 2 so 4 (sulfuric acid), naoh ( sodium hydroxide) and koh (potassium hydroxide) are all strong electrolytes.
 Source: clutchprep.com
Source: clutchprep.com
These ions are good conductors of electric current in the solution. Solution for which of the following is considered a strong electrolyte? Weak electrolytes include weak acids, weak bases, and a variety of other compounds.
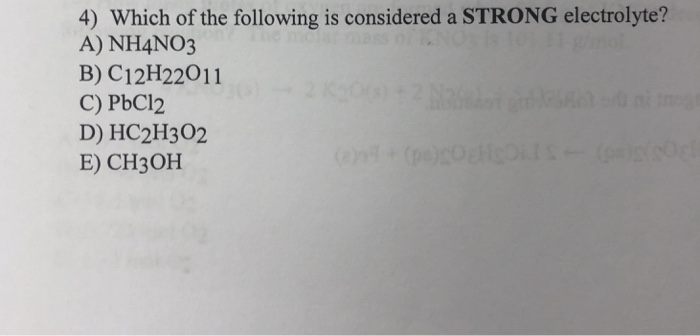
A) nh4no3 b) pbcl2 c) hc2h3o2 d) ch3oh e) c12h22o11 a) nh4no3 b) pbcl2 c) hc2h3o2 d) ch3oh e) c12h22o11 A strong electrolyte is a solution/solute that completely, or almost completely, ionizes or dissociates in a solution. However, some electrolytes do not completely dissolve in water but are considered as strong electrolytes.
 Source: numerade.com
Source: numerade.com
A) nh4no3 b) pbcl2 c) hc2h3o2 d) ch3oh e) c12h22o11 a) nh4no3 b) pbcl2 c) hc2h3o2 d) ch3oh e) c12h22o11 Hope this answers the question. Of the following , the strong electrolyte would be nh4no3.
 Source: chegg.com
Source: chegg.com
Hcl (hydrochloric acid), h 2 so 4 (sulfuric acid), naoh ( sodium hydroxide) and koh (potassium hydroxide) are all strong electrolytes. That is, the principal species in solution for strong electrolytes are ions, while the principal specie in. N h 4 c l (strong electrolyte) c.
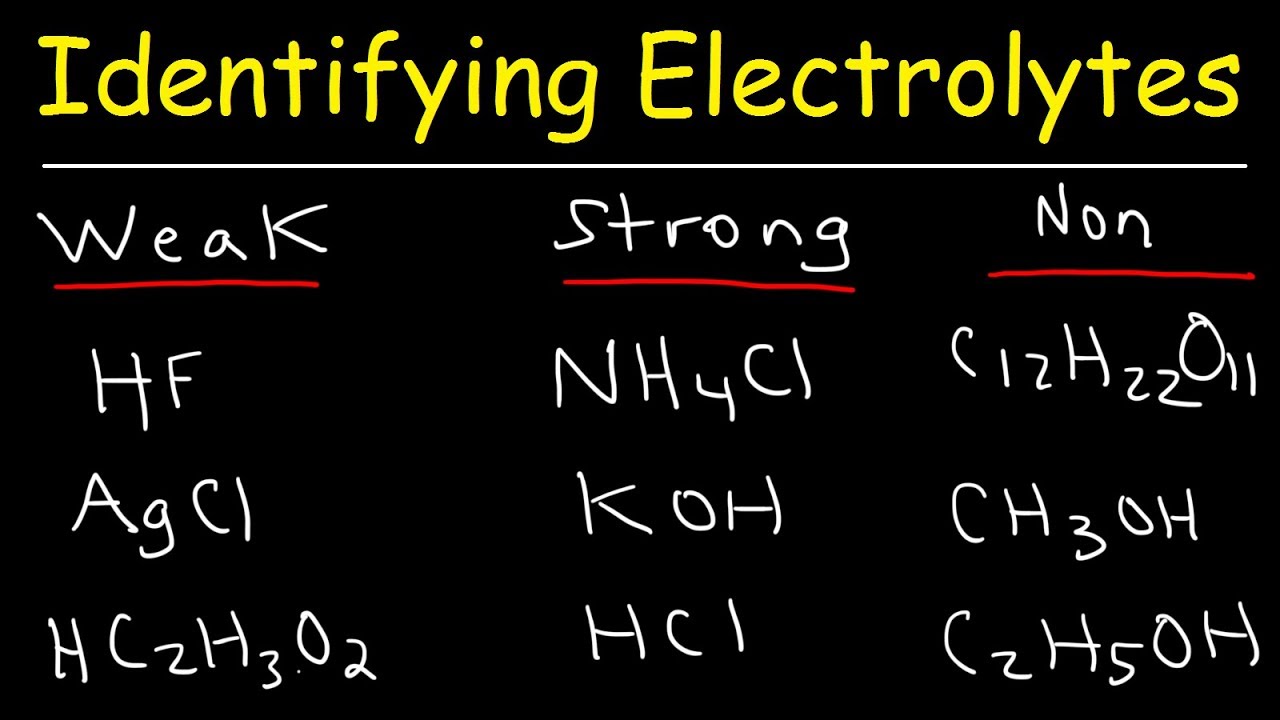 Source: youtube.com
Source: youtube.com
Learn this topic by watching electrolytes concept videos. Common strong electrolytes are strong acids, strong bases, and ionic salts. Electrolytes are essential for basic life functioning, such as maintaining electrical neutrality in cells, generating and conducting action potentials in the nerves and muscles.
Also Read :





