A) carbon (atomic number 6) b) neon (atomic number 10) c) oxygen (atomic number 8) d) sodium (atomic number 11) Which of the following is chemically inert (unreactive)?
Which Of The Following Is Chemically Inert Unreactive. 3 🔴 on a question which of the following is chemically inert (unreactive)? Most group 8 or 18 elements that appear in the last column of the periodic table (helium, neon, argon, krypton, xenon and radon) are classified as inert (or unreactive). Any of the gaseous elements helium, neon, argon, krypton, xenon, and radon, occupying group 0 (18) of the periodic table. The chemically inert element is neon.
 Apa Arti "Chemically Inert" Dalam Bahasa Indonesia From tr-ex.me
Apa Arti "Chemically Inert" Dalam Bahasa Indonesia From tr-ex.me
Related Post Apa Arti "Chemically Inert" Dalam Bahasa Indonesia :
Nitrogen, the unreactive gas on the other hand, nitrogen is not a noble gas. Which of the following is chemically inert (unreactive)? 3 letter words wan 4 letter words What are all the unreactive elements?
Asked feb 6, 2017 in trades & technology by hoshgosh.
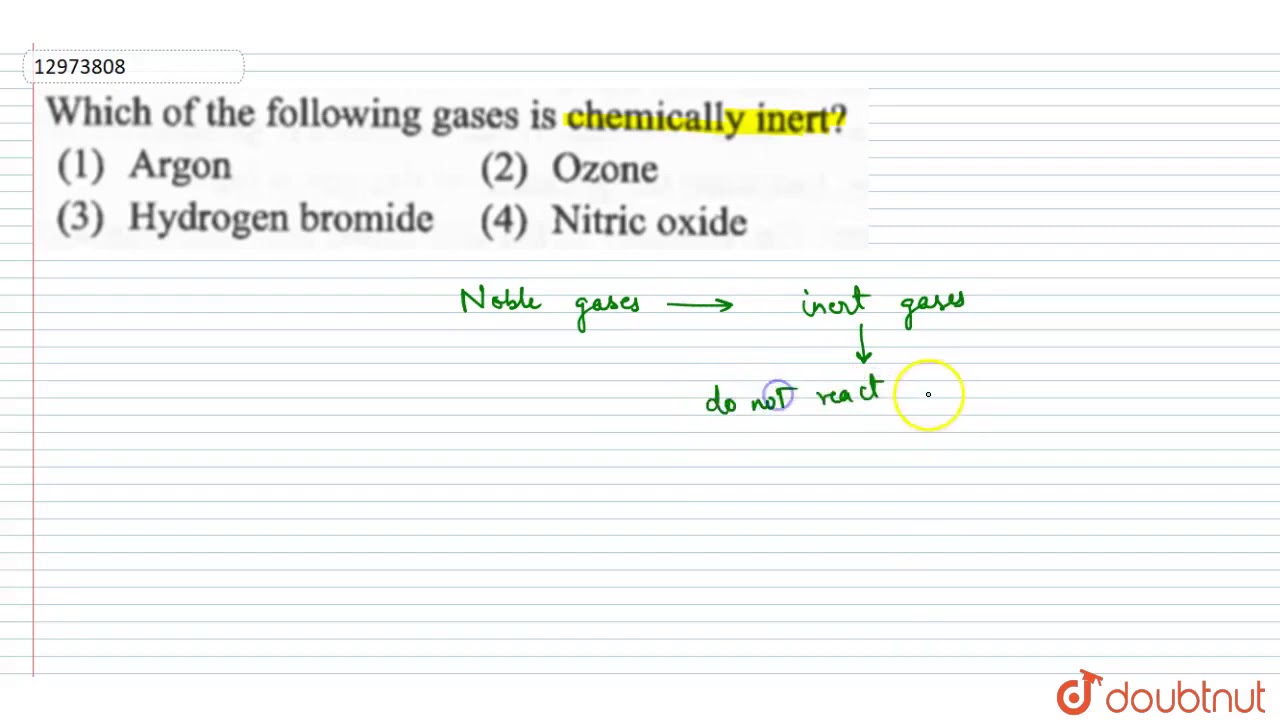
Is chemically inert in almost all environments? As adjectives the difference between inert and unreactive Most group 8 or 18 elements that appear in the last column of the periodic table (helium, neon, argon, krypton, xenon and radon) are classified as inert (or unreactive). Argon is the cheapest noble gas and thus the most frequently used. Which of the following is chemically inert (unreactive)? Add your answer and earn points.
 Source: breakingatom.com
Source: breakingatom.com
They show trends in their physical properties. Asked feb 6, 2017 in trades & technology by hoshgosh. Reactivity of an element is defined as the tendency of an element to loose or gain electrons.
 Source: tr-ex.me
Source: tr-ex.me
Dinitrogen in chemically unreactive at ordinary temperature and is very stable. Why is ceramic chemically inert? Which of the following is chemically inert (unreactive)?
 Source: slidetodoc.com
Source: slidetodoc.com
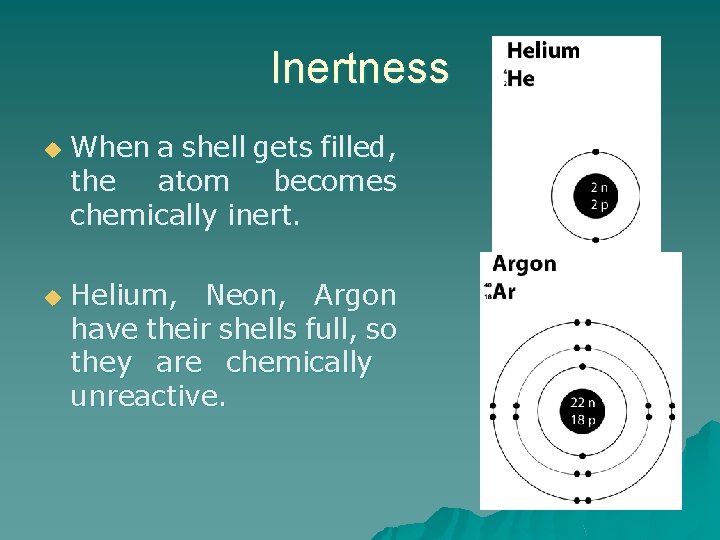
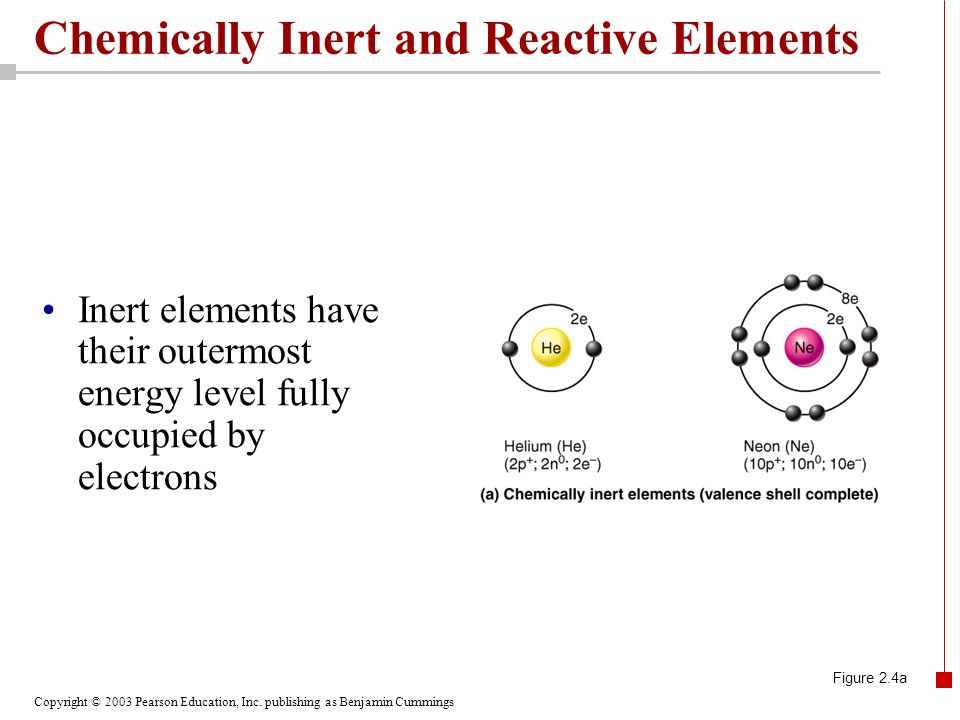
Most group 8 or 18 elements that appear in the last column of the periodic table (helium, neon, argon, krypton, xenon and radon) are classified as inert (or unreactive). The chemically inert element is neon. These elements are stable in their naturally occurring form (gaseous form) and.
 Source: slideplayer.com
Source: slideplayer.com
From a thermodynamic perspective, a substance is inert, or nonlabile, if it is thermodynamically unstable yet decomposes at a slow, or negligible rate. Reactivity of an element is defined as the tendency of an element to loose or gain electrons. A) 14 electrons b) 14 neutrons c) 10 neutrons d) 24 protons.
 Source: slidetodoc.com
Source: slidetodoc.com
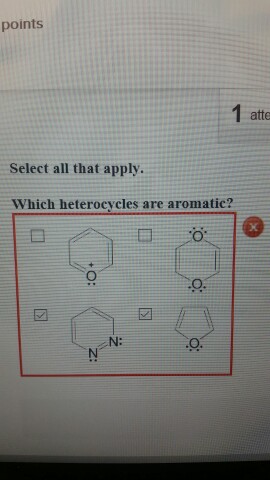
- which of the following is chemically inert (unreactive)? Due to this rapid quenching, they do not get enough time to form proper bonds and the bonds which were able to form in that time, become quiet hard due to the rapid processing. 48) which of the following is chemically inert (unreactive)?
 Source: quizlet.com
Source: quizlet.com
In context|chemistry|lang=en terms the difference between inert and unreactive is that inert is (chemistry) a substance that does not react chemically while unreactive is (chemistry) not reactive; (a) carbon (atomic number 6) (b) sodium (atomic number 11) (c) neon (atomic number 10) (d) oxygen (atomic number 8) A) 14 electrons b) 14 neutrons c) 10 neutrons d) 24 protons.
 Source: slidetodoc.com
Source: slidetodoc.com
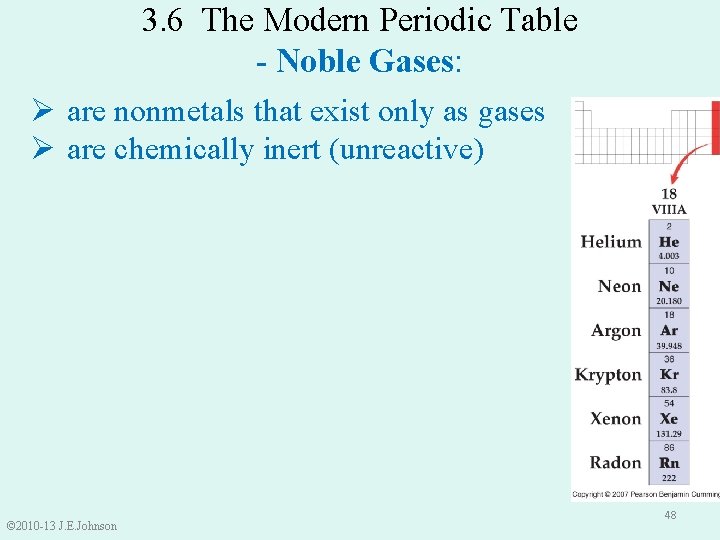
These are elements that does not react chemically, elements found in group 8 of the periodic table are enlisted here such as the xenon, argon, neon, krypton, radon. To be chemically unreactive however like most noble gases except for in rare cases, xenon which can react with oxygen, it means it is inert or chemically unreactive. A) carbon (atomic number 6) b) neon (atomic number 10) c) oxygen (atomic number 8) d) sodium (atomic number 11) b) neon (atomic number 10) an atom with an atomic number of 10 and a mass number of 24 would have _____.
 Source: chemistryfromscratch.org
Source: chemistryfromscratch.org
Helium is unreactive and is chemically inert. 3 letter words wan 4 letter words A) carbon (atomic number 6) b) neon (atomic number 10) c) oxygen (atomic number 8) d) sodium (atomic number 11) b) neon (atomic number 10) an atom with an atomic number of 10 and a mass number of 24 would have _____.
 Source: slideplayer.com
Source: slideplayer.com
Which of the following is chemically inert (unreactive)? That means that it is stable. Reactivity of an element is defined as the tendency of an element to loose or gain electrons.
 Source: youtube.com
Source: youtube.com
The name comes from the fact that these elements are virtually unreactive towards other elements or compounds. Add your answer and earn points. Argon is the cheapest noble gas and thus the most frequently used.
 Source: study.com
Source: study.com
Why is ceramic chemically inert? Reactivity of an element is defined as the tendency of an element to loose or gain electrons. Two nitrogen atoms make up the nitrogen molecule (n2), so it has no free electrons like argon and thus the same properties of a noble gas under nearly all uses.
 Source: coursehero.com
Source: coursehero.com
Krypton generally compounds with fluorine such as krf2, krf4. To be chemically unreactive however like most noble gases except for in rare cases, xenon which can react with oxygen, it means it is inert or chemically unreactive. Which of the following is chemically inert (unreactive)?
 Source: ilpi.com
Source: ilpi.com
In chemistry, the term chemically inert is used to describe a substance that is not chemically reactive. A) sodium (atomic number 11) b) oxygen (atomic number 8) c) carbon (atomic number 6) d) neon. Any of the gaseous elements helium, neon, argon, krypton, xenon, and radon, occupying group 0 (18) of the periodic table.
 Source: coursehero.com
Source: coursehero.com
Add your answer and earn points. Synonyms, crossword answers and other related words for chemically unreactive [inert] we hope that the following list of synonyms for the word inert will help you to finish your crossword today. Radon is a chemically inert gas that emanates from certain soils and builds up in confined spaces such as the basements of buildings.
 Source: slidetodoc.com
Source: slidetodoc.com
Reactivity of an element is defined as the tendency of an element to loose or gain electrons. What are all the unreactive elements? We�ve arranged the synonyms in length order so that they are easier to find.
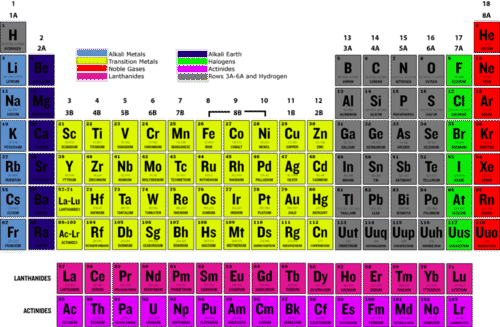
Helium is unreactive and is chemically inert. Two nitrogen atoms make up the nitrogen molecule (n2), so it has no free electrons like argon and thus the same properties of a noble gas under nearly all uses. Radon is a chemically inert gas that emanates from certain soils and builds up in confined spaces such as the basements of buildings.
 Source: knowswhy.com
Source: knowswhy.com
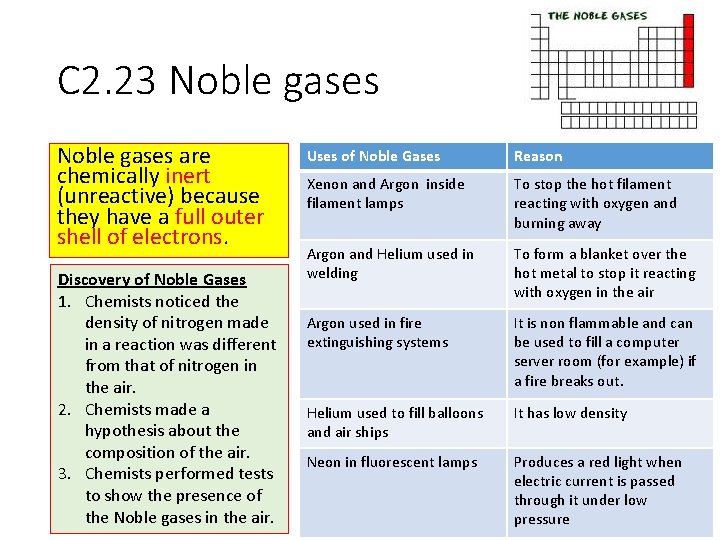
To be chemically unreactive however like most noble gases except for in rare cases, xenon which can react with oxygen, it means it is inert or chemically unreactive. 48) which of the following is chemically inert (unreactive)? Elements are divided into various categories:
 Source: slidetodoc.com
Source: slidetodoc.com
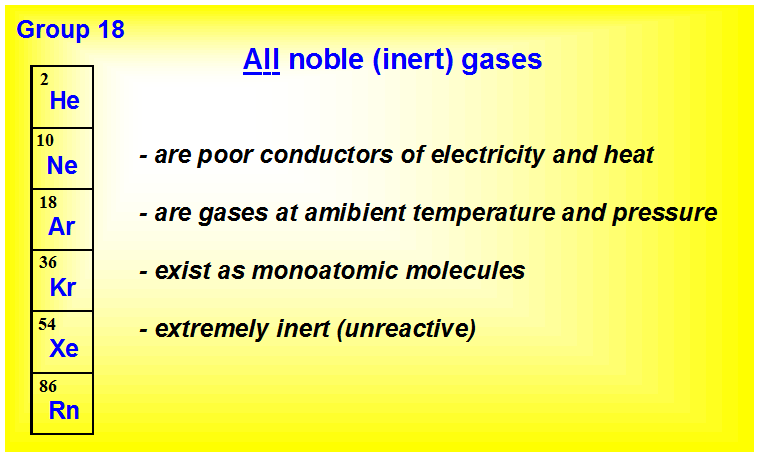
Group 18 are the noble gases. Asked feb 6, 2017 in trades & technology by hoshgosh. 3 🔴 on a question which of the following is chemically inert (unreactive)?
 Source: socratic.org
Source: socratic.org
3 letter words wan 4 letter words Group 8a (or viiia) of the periodic table are the noble gases or inert gases: 48) which of the following is chemically inert (unreactive)?

When a compound or molecule reaches a valence electrons totaling 8 or 16 or 24. They are typically chemically inert and do not react with other materials. Group 18 are the noble gases.
Also Read :