View solution > identify the type of chemical reaction taking place in each of the following : Therefore, respiration and burning of a candle are examples of exothermic reaction whereas evaporation of water and melting of ice are examples of endothermic reaction.
Which Of The Following Is An Exothermic Reaction. C a l c i c i u m o x i d e (q u i c k l i m e) c a o (s) + w a t e r h 2 o (l) → c a l c i u m h y d r o x i d e (s l a k e d l i m e) c a (o h. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. When energy is transferred as heat from the surroundings to the system, δh is negative.e. As a result, the molecules are at a lower final energy state after releasing the energy to the surroundings.
 Which One Of The Following Is An Endothermic Reaction ? - Youtube From youtube.com
Which One Of The Following Is An Endothermic Reaction ? - Youtube From youtube.com
Related Post Which One Of The Following Is An Endothermic Reaction ? - Youtube :
Since every chemical change involves a gain or loss of energy. Similarly, when an acid or a base is diluted with water, heat is released making it a highly exothermic reaction. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. Generally, decomposition reactions are endothermic reactions.
Therefore, it can be understood that the net amount.
D.)the longest arrow represents the. Exothermic reaction means exo meaning releases and thermic means heat. Which of the following statements are true? B.)there are two intermediate reactions in this system. During this reaction, a large amount of heat is released. Which of the following statements is true?
 Source: chegg.com
Source: chegg.com
All of the above are true. The following reactions are exothermic in an exothermic reaction, heat has been released to the surroundings from the system. Example of a temperature change that might occur in an exothermic reaction.
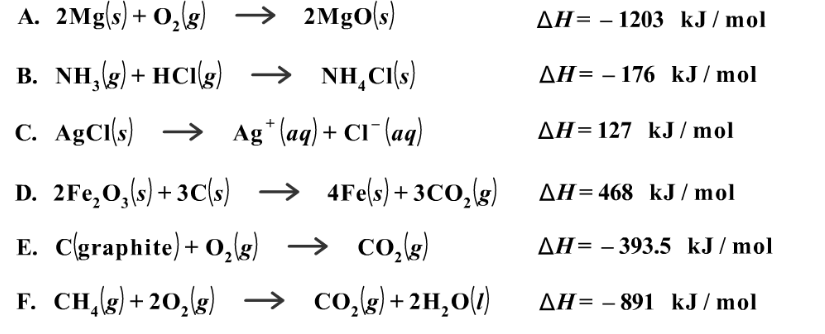 Source: clutchprep.com
Source: clutchprep.com
A few examples are neutralization, burning a substance, reactions of fuels, deposition of dry ice, respiration, solution of sulfuric acid into water and much more. It is neither endothermic nor exothermic. The exothermic reaction is the opposite.
 Source: teachoo.com
Source: teachoo.com
The exothermic reaction is the opposite. A reaction which absorbs energy is endothermic. Which of the following is true of the reaction:
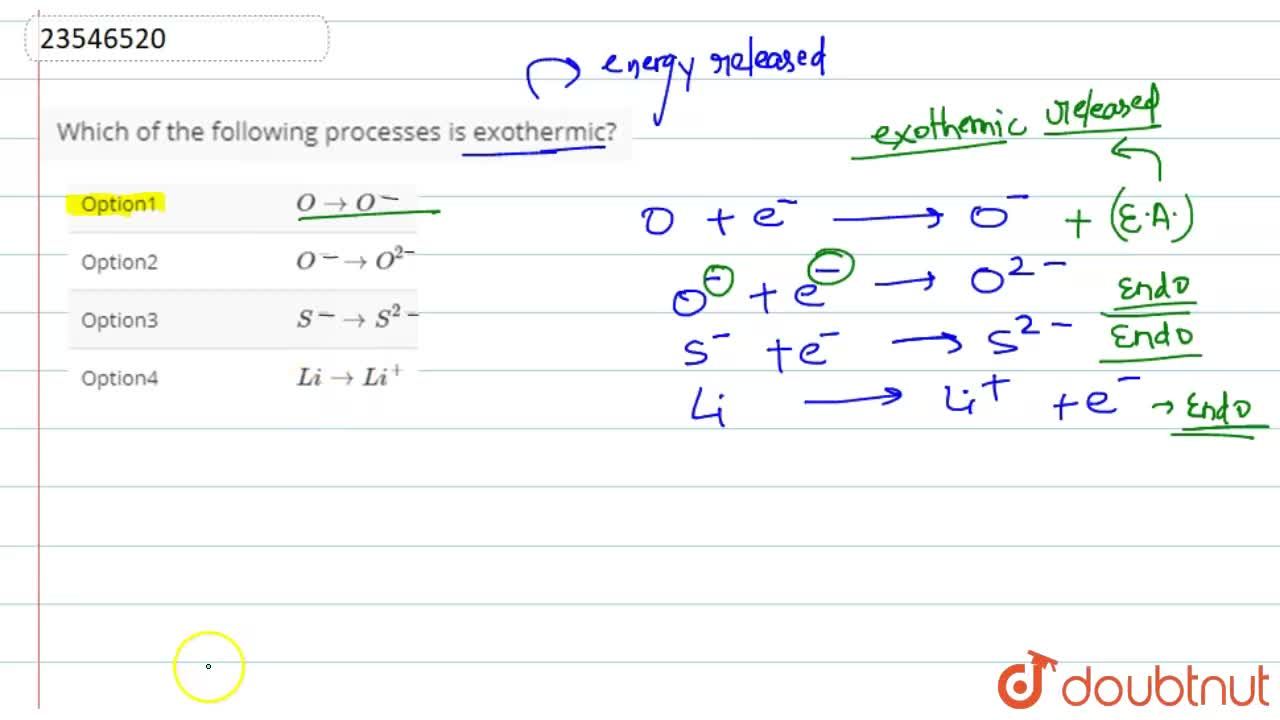 Source: doubtnut.com
Source: doubtnut.com
When energy is transferred as heat from the system to surroundings, δh is negative.d. The following reactions are exothermic in an exothermic reaction, heat has been released to the surroundings from the system. Which of the following statements is true?
 Source: youtube.com
Source: youtube.com
Therefore, respiration and burning of a candle are examples of exothermic reaction whereas evaporation of water and melting of ice are examples of endothermic reaction. All of the above are true. It is neither endothermic nor exothermic.
 Source: toppr.com
Source: toppr.com
In an exothermic reaction, change in enthalpy ( δh) will be negative. The exothermic reaction is the opposite of an endothermic reaction. B.)there are two intermediate reactions in this system.
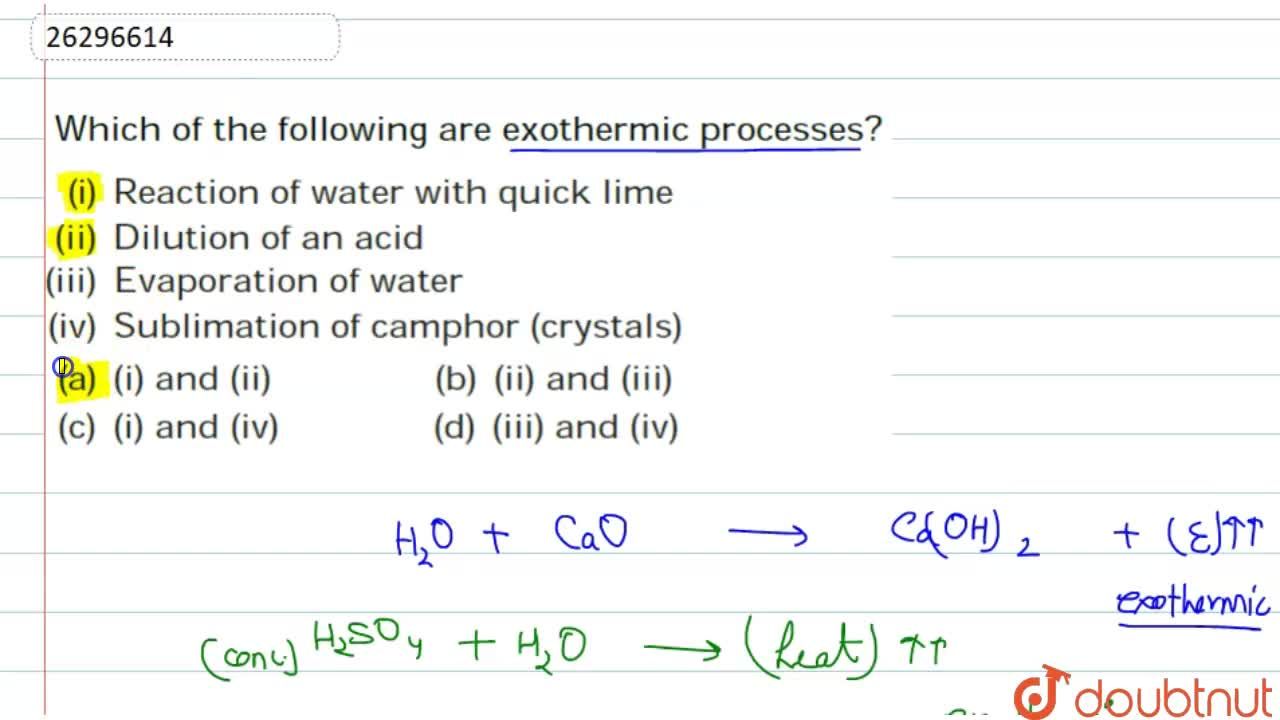 Source: doubtnut.com
Source: doubtnut.com
Example of a temperature change that might occur in an exothermic reaction. A.)the overall chemical reaction is exothermic. It is neither endothermic nor exothermic.

In an exothermic reaction, heat is released. Therefore, respiration and burning of a candle are examples of exothermic reaction whereas evaporation of water and melting of ice are examples of endothermic reaction. Since every chemical change involves a gain or loss of energy.
 Source: thoughtco.com
Source: thoughtco.com
In an endothermic reaction, heat is absorbed. Which of the following sentences best describes the energy change of photosynthesis? A photosynthesis is an endothermic reaction, so more energy is absorbed making bonds than is released breaking bonds.
 Source: youtube.com
Source: youtube.com
Therefore, respiration and burning of a candle are examples of exothermic reaction whereas evaporation of water and melting of ice are examples of endothermic reaction. Therefore, it is an exothermic reaction. In an exothermic reaction, change in enthalpy ( δh) will be negative exothermic reactions.
 Source: youtube.com
Source: youtube.com
Similarly, when an acid or a base is diluted with water, heat is released making it a highly exothermic reaction. Generally, decomposition reactions are endothermic reactions. None of these, the reaction cannot occur.
 Source: youtube.com
Source: youtube.com
This reaction releases heat, hence, an exothermic reaction. Melting ice is an endothermic process. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction.

Δh for an exothermic reaction is positive.c. When energy is transferred as heat from the surroundings to the system, delta h is negative. As a result, the molecules are at a lower final energy state after releasing the energy to the surroundings.
 Source: toppr.com
Source: toppr.com
C.)the third intermediate reaction is endothermic. Which of the following is true of the reaction: An endothermic process requires the absorption of.
 Source: brainly.com
Source: brainly.com
Therefore, it can be understood that the net amount. Generally, decomposition reactions are endothermic reactions. The evaporation of water is an exothermic process.f.
 Source: clutchprep.com
Source: clutchprep.com
It is neither endothermic nor exothermic. Delta h for an endothermic reaction is positive delta h for an exothermic reaction is positive when energy is transferred as heat from the system to the surroundings, delta h is negative. Reactants → products + energy.
 Source: slideserve.com
Source: slideserve.com
This reaction releases heat, hence, an exothermic reaction. Which of the following sentences best describes the energy change of photosynthesis? Which of the following is true of the reaction:
 Source: toppr.com
Source: toppr.com
A reaction which releases energy is exothermic. This reaction releases heat, hence, an exothermic reaction. Which of the following sentences best describes the energy change of photosynthesis?
 Source: youtube.com
Source: youtube.com
In an endothermic reaction, heat is absorbed. Which of the following is an exothermic reaction? Exothermic reaction means exo meaning releases and thermic means heat.
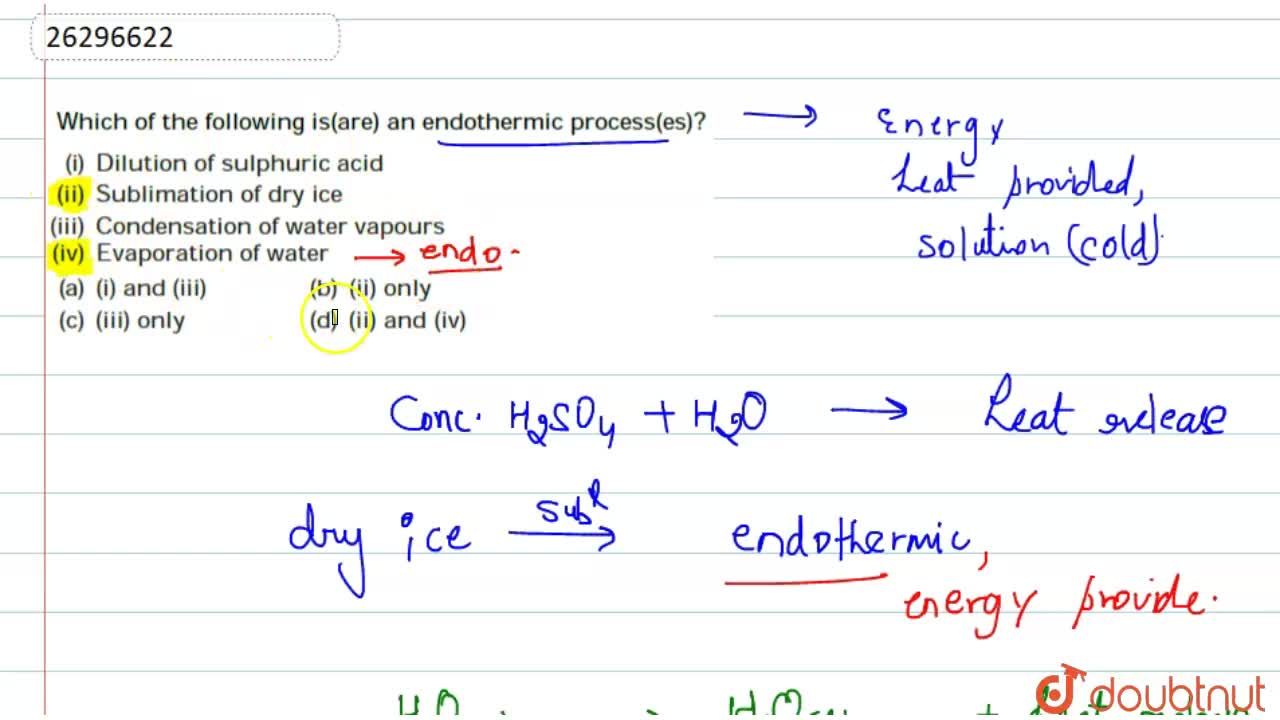 Source: doubtnut.com
Source: doubtnut.com
The evaporation of water is an exothermic process.f. This is essentially a corrosion reaction in which the metallic bonds in fe are broken and new bonds between fe and o are formed, this is. In an exothermic reaction, change in enthalpy ( δh) will be negative exothermic reactions.
Also Read :