The example for arrhenius base is highly soluble sodium hydroxide compound in water, which dissociates to give sodium ion and hydroxide ion. Indicate whether each of the following statements is characteristics of an arrhenius acid, arrhenius base, or both:
Which Of The Following Is An Arrhenius Base. Which of the following compounds is not an arrhenius acid? Which of the following compounds is a strong arrhenius base? To recognize the arrhenius base look for a molecule ending in oh, but not following chx which refers to an alcohol. The example for arrhenius base is highly soluble sodium hydroxide compound in water, which dissociates to give sodium ion and hydroxide ion.
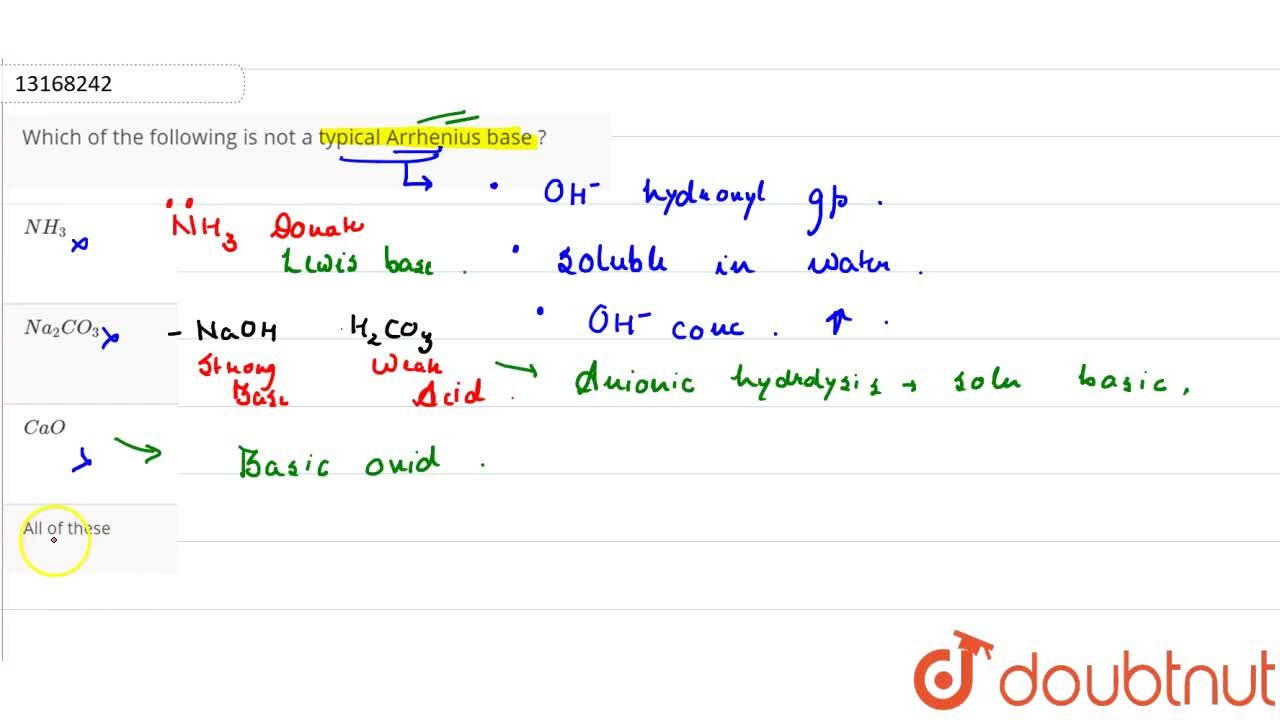 Which Of The Following Is Not A Typical Arrhenius Base ? From doubtnut.com
Which Of The Following Is Not A Typical Arrhenius Base ? From doubtnut.com
Related Post Which Of The Following Is Not A Typical Arrhenius Base ? :
A possible reaction leading to removal of clo from the atmosphere is 2clo → cl2 + o2. Arrhenius bases raise the hydroxide ion concentration when dissolved in water. What are properties of a base? Similarly, arrhenius defined a base as a compound that increases the concentration of hydroxide ion (oh −) in aqueous solution.
Ch3co2h is acetic acid and it gives h+ in aqueous solution so it is arrhenius acid.
Which of the following compounds is a strong arrhenius base? Which of the following compounds is not an arrhenius acid? 43) which one of the following compounds behaves as an acid when dissolved in water? Further explanation acids and bases are two classes of chemicals that are very commonly found around us. What makes an arrhenius base? Similarly, arrhenius defined a base as a compound that increases the concentration of hydroxide ion (oh −) in aqueous solution.
 Source: clutchprep.com
Source: clutchprep.com
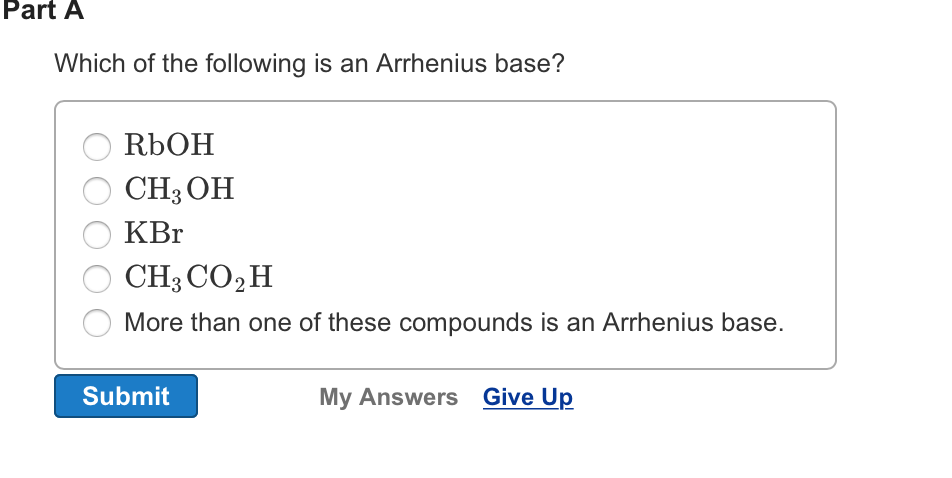
Which of the following is an arrhenius base? Acids produce hydroxide ions in aqueous solution and bases produce hydrogen ions. H3oh nabr ch3co2h lioh more than one of these compounds is an arrhenius base.

- which of the following compounds is an arrhenius base? >>which of the following is an arrhenius b. Which of the following is an arrhenius base?
 Source: chegg.com
Source: chegg.com
• an arrhenius acid increases the concentration of h+ ion when dissolved. Similarly, arrhenius defined a base as a compound that increases the concentration of hydroxide ion (oh −) in aqueous solution. Sodium hydroxide (naoh) is an arrhenius base, as naoh is an ionic compound that dissociates into na + and oh− ions when dissolved in water.
 Source: doubtnut.com
Source: doubtnut.com
Identify each of the following as an arrhenius acid, arrhenius base, or none: Ch₃cooh → ch₃coo⁻ + h⁺ therefore, it can act as arrhenius acid instead of arrhenius base. To recognize the arrhenius base look for a molecule ending in oh, but not following chx which refers to an alcohol.

Acids produce hydrogen ions in aqueous solution and bases produce hydroxide ions. A) ch3co2h b) koh c) ch3oh d) licl e) more than one of these compounds is an arrhenius base. Has a slippery feel d.
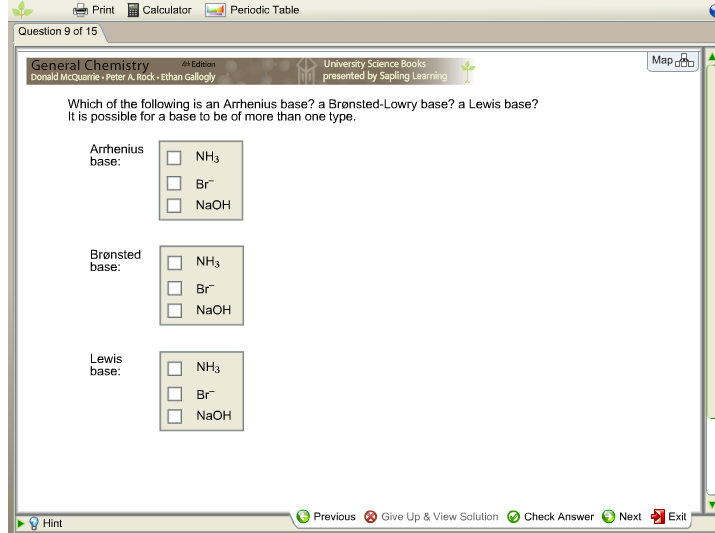 Source: chegg.com
Source: chegg.com
Acetic acid when dissolved in water produces h⁺ ions and acetate ions i.e. Acids produce hydrogen ions in aqueous solution and bases produce hydroxide ions. Bases taste bitter, feel slippery, and conduct electricity when dissolved in water.
 Source: slideplayer.com
Source: slideplayer.com
Further explanation acids and bases are two classes of chemicals that are very commonly found around us. We’re being asked to determine which of the following given compounds is an arrhenius base. A) ch3co2h b) ch3ch2nh2 c) hno2 d) h2so4
 Source: sciencenotes.org
Source: sciencenotes.org
Acids produce hydrogen ions in aqueous solution and bases produce hydroxide ions. D) more than one correct response. Acetic acid when dissolved in water produces h⁺ ions and acetate ions i.e.
 Source: study.com
Source: study.com
H3oh nabr ch3co2h lioh more than one of these compounds is an arrhenius base. 42) which of the following compounds is an arrhenius base? Ch3co2h is acetic acid and it gives h+ in aqueous solution so it is arrhenius acid.
 Source: ck12.org
Source: ck12.org
To recognize the arrhenius base look for a molecule ending in oh, but not following chx which refers to an alcohol. What makes an arrhenius base? Similarly, arrhenius defined a base as a compound that increases the concentration of hydroxide ion (oh −) in aqueous solution.
 Source: chegg.com
Source: chegg.com
Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield electrically charged atoms or molecules, called ions, one of which is a hydrogen ion (h+), and that bases ionize in water to yield hydroxide ions (oh−). Bases taste bitter, feel slippery, and conduct electricity when dissolved in water. A possible reaction leading to removal of clo from the atmosphere is 2clo → cl2 + o2.
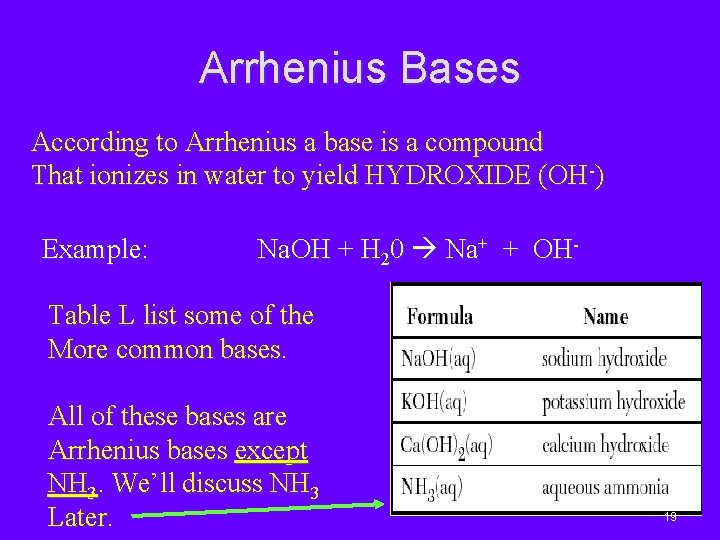 Source: slidetodoc.com
Source: slidetodoc.com
- which of the following compounds is an arrhenius base? Which of the following is an arrhenius base? Acids are electron donors and bases.
 Source: doubtnut.com
Source: doubtnut.com
- which one of the following compounds behaves as an acid when dissolved in water? Acids are electron donors and bases. 43) which one of the following compounds behaves as an acid when dissolved in water?
 Source: slideplayer.com
Source: slideplayer.com
Sodium hydroxide (naoh) is an arrhenius base, as naoh is an ionic compound that dissociates into na + and oh− ions when dissolved in water. Identify each of the following as an arrhenius acid, arrhenius base, or none: Arrhenius theory, theory, introduced in 1887 by the swedish scientist svante arrhenius, that acids are substances that dissociate in water to yield electrically charged atoms or molecules, called ions, one of which is a hydrogen ion (h+), and that bases ionize in water to yield hydroxide ions (oh−).
 Source: chegg.com
Source: chegg.com
For a substance to be classified as an arrhenius base, it must produce both hydroxide ions and hydorgen ions in solution. Acids are proton (h +) acceptors and bases are proton donors. What are properties of a base?
 Source: intechopen.com
Source: intechopen.com
What makes an arrhenius base? Ch3co2h is acetic acid and it gives h+ in aqueous solution so it is arrhenius acid. Indicate whether each of the following statements is characteristics of an arrhenius acid, arrhenius base, or both:
 Source: chegg.com
Source: chegg.com
The example for arrhenius base is highly soluble sodium hydroxide compound in water, which dissociates to give sodium ion and hydroxide ion. H3oh nabr ch3co2h lioh more than one of these compounds is an arrhenius base. >>which of the following is an arrhenius b.
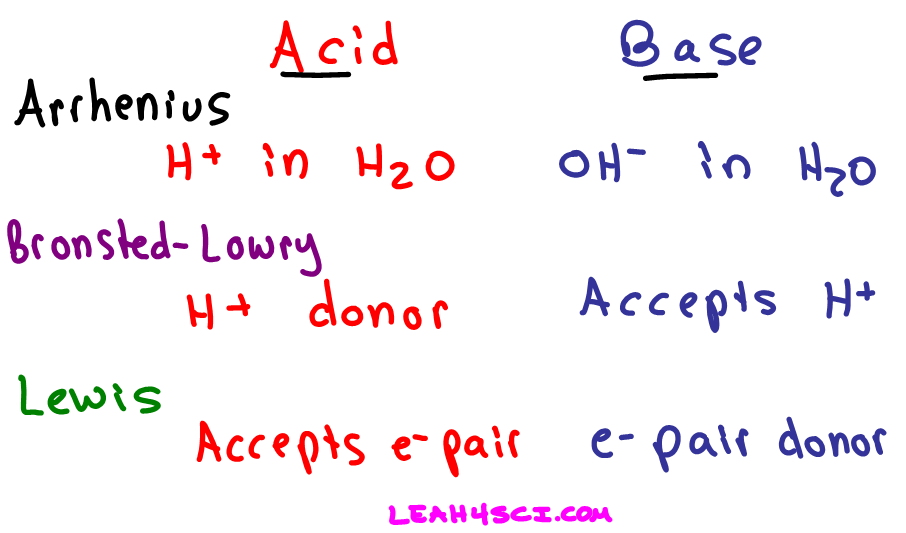 Source: leah4sci.com
Source: leah4sci.com
Has a slippery feel d. • an arrhenius acid increases the concentration of h+ ion when dissolved. Arrhenius defined an acid as a compound that increases the concentration of hydrogen ion (h +) in aqueous solution.
 Source: numerade.com
Source: numerade.com
To recognize the arrhenius base look for a molecule ending in oh, but not following chx which refers to an alcohol. An arrhenius base is a substrate that increases the concentration of hydroxide ions in the aqueous solution. Acids are proton (h +) acceptors and bases are proton donors.
 Source: study.com
Source: study.com
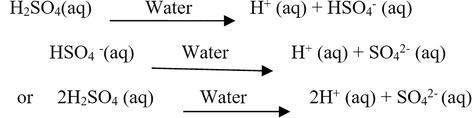
Ch3co2h is acetic acid and it gives h+ in aqueous solution so it is arrhenius acid. Arrhenius acids include sulfuric acid (h2so4), hydrobromic acid (hbr), and nitric acid (hno3). Arrhenius bases raise the hydroxide ion concentration when dissolved in water.
Also Read :