These include combustion, rusting, photosynthesis, respiration and decomposition. Here, no change of oxidation state takes place.
Which Of The Following Is A Redox Reaction. So, also a change in oxidation number there, for this is a redox reaction reaction. Which of the following is true about a redox reaction? Redox reactions are chemical reactions involving oxidation and reduction occurring simultaneously. Cu + s → cus
 Which One Of The Following Is A Redox Reaction ? From toppr.com
Which One Of The Following Is A Redox Reaction ? From toppr.com
Related Post Which One Of The Following Is A Redox Reaction ? :
Which of the following reactions is a redox reaction? Which of the following is a redox reaction ? Here, no change of oxidation state takes place. Which of the following is redox reaction?
You see english choices, you place currency and to so in this case,.
Which of the following are redox reactions? So, also a change in oxidation number there, for this is a redox reaction reaction. Which of the following are redox reactions? If we looks at the reaction; Answers (1) redox reaction as a class of reactions in which oxidation and reduction reactions occur simultaneously. Redox reaction is that reaction in which both oxidation and reduction of a chemical species takes place at a time.
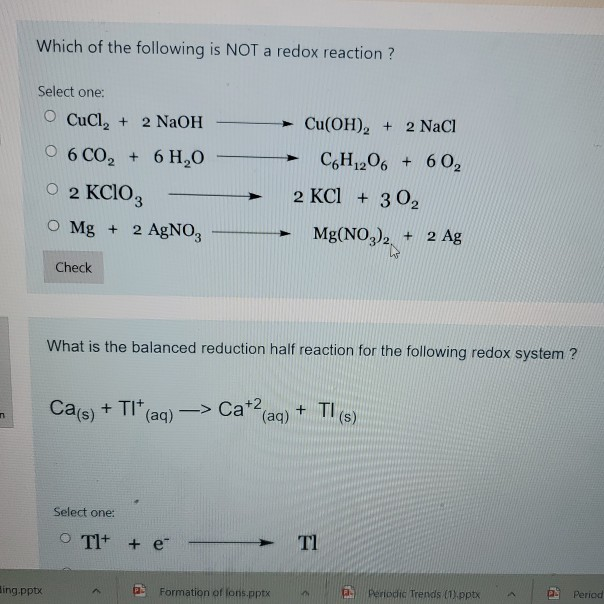 Source: chegg.com
Source: chegg.com
Which of the following are redox reactions? If there is no change in oxidation number, then the reaction is not a redox reaction. Reduction is when an atom gains an electron or electrons.
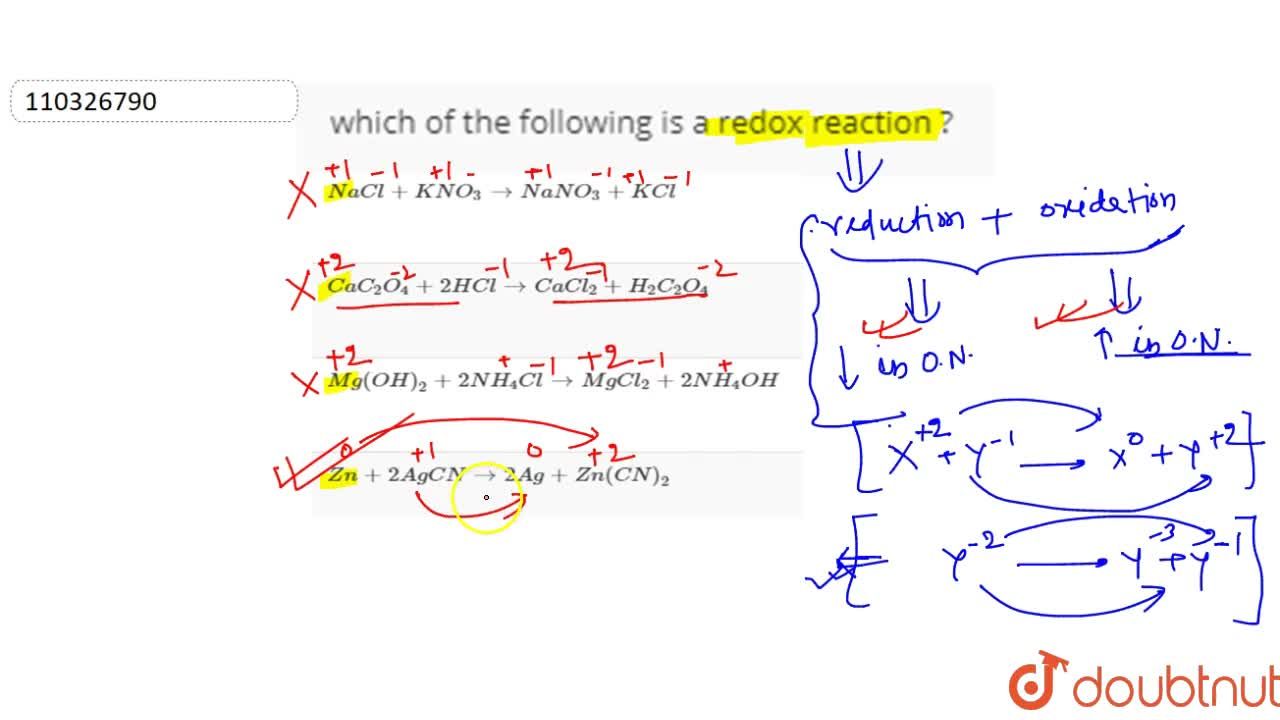 Source: doubtnut.com
Source: doubtnut.com
In the other options (a), (b) and (d) there is no change in oxidation state of any atom, hence these are not redox reaction. So3(g) + h2o(l) > h2so4(aq) there was no change in the oxidation numbers of species from left to right of the reaction equation. Which of the following is redox reaction?
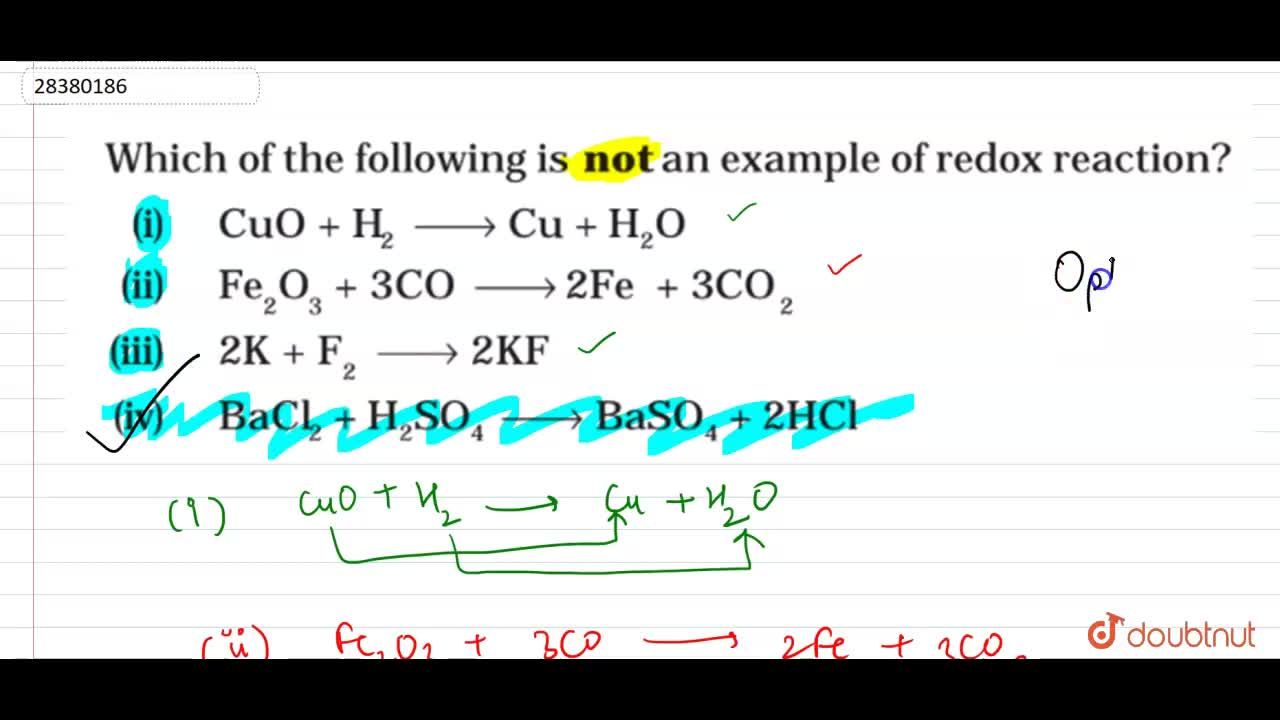 Source: doubtnut.com
Source: doubtnut.com
Reduction is when an atom gains an electron or electrons. Redox reaction is that reaction in which both oxidation and reduction of a chemical species takes place at a time. So the correct answer is option (d).
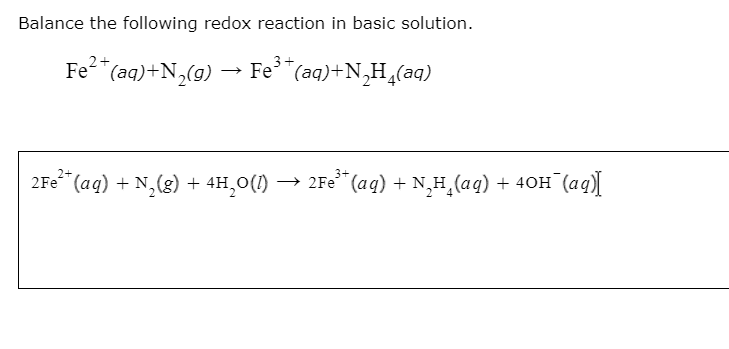 Source: oneclass.com
Source: oneclass.com
A redox reaction is defined as a chemical reaction that involves both oxidation and reduction. The oxidation state of clo is +7. In oxidation process, there is an increase in oxidation state of an atom, ion.
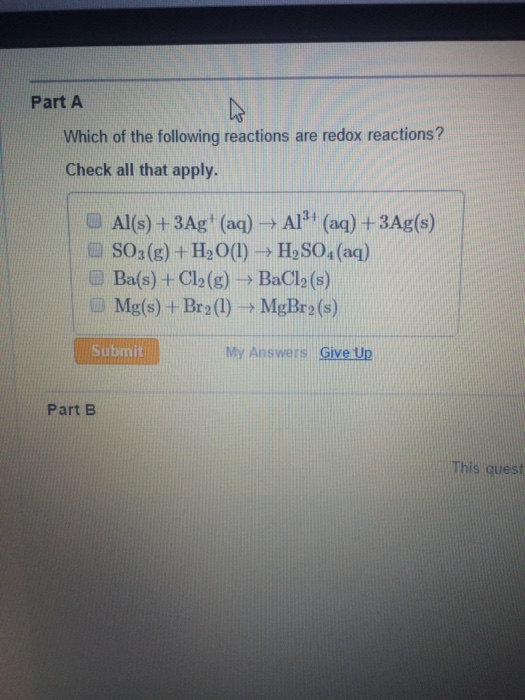
Reduction is when an atom gains an electron or electrons. All the processes given above are redox processes as in electrochemical process like extraction of highly reactive metals is a redox reaction. Which of the following reactions is a redox reaction(can be more than one)?show with oxidation numbers.
 Source: toppr.com
Source: toppr.com
If there is no change in oxidation number, then the reaction is not a redox reaction. I�m jewish nice plush toys and it�s called sale museum this year. B) the products are the constituent elements.
 Source: chegg.com
Source: chegg.com
To identify a redox reaction, we must first calculate the oxidation number of each atom in the reaction. Answers (1) redox reaction as a class of reactions in which oxidation and reduction reactions occur simultaneously. Gain of electron or decrease in oxidation state.
 Source: neetlab.com
Source: neetlab.com
A redox reaction is one in which there is a change in the oxidation number of the reactants from left to right of the reaction equation. A) 2 na + 2 h2o à 2 naoh + h2 / c) 2 k + f2 à 2 kf b) mgbr2 + 2 naf à mgf2 + 2 nabr / d) ch4 + 2 o2 à co2 + 2 h2o. Of none of the atoms undergo a change and therefore, this is not a redox reaction.
 Source: toppr.com
Source: toppr.com
Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of rules formulated on the basis that electron pair in a covalent bond belongs entirely to more electronegative elements. The given options are clo, no,so, io. We have two waters reacting with each other, forming two hydrogen and two oxygen�s using the oxidation of the real oxygen minus two.
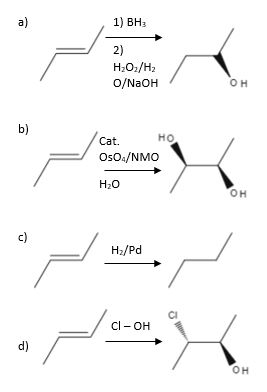 Source: study.com
Source: study.com
Which of the following are redox reactions? To identify a redox reaction, we must first calculate the oxidation number of each atom in the reaction. Here, zn0is oxidized to zn+2and ag+1 is reduced to ag0.
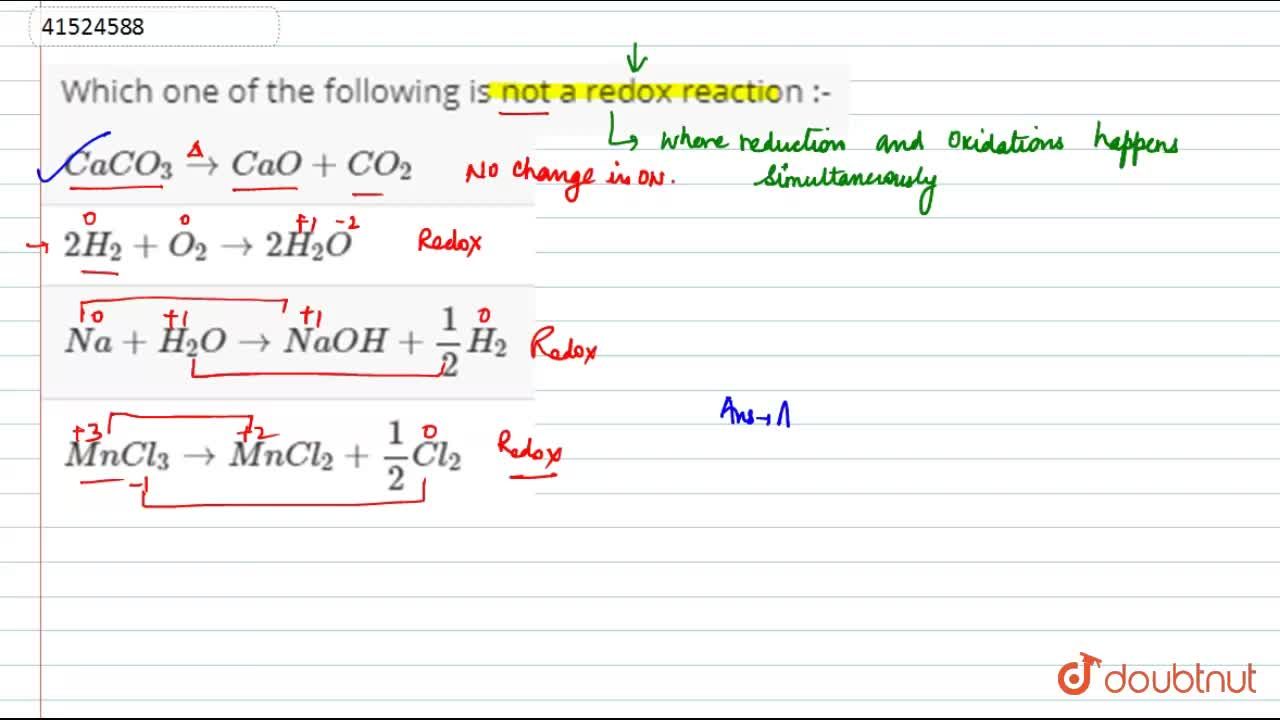 Source: doubtnut.com
Source: doubtnut.com
So the correct answer is option (d). Which of the following is true about a redox reaction? Redox reaction is that reaction in which both oxidation and reduction of a chemical species takes place at a time.

So, also a change in oxidation number there, for this is a redox reaction reaction. We can find out the redox reaction if there has been a transfer of electrons means there should be change in the oxidation number of the reactants and the products. If one element has a decrease in its oxidation state, the other element has an increase in its oxidation state.
 Source: youtube.com
Source: youtube.com
If there is a change in oxidation number, then the reaction is a redox reaction. The given options are clo, no,so, io. B) the products are the constituent elements.

Cu0 + 2agno3+1 → cu(no3)2+2 + 2ag0this reaction is an example of redox reaction in which cu is being oxidised to cu2+ while ag+ is getting reduced to ag. If there is a change in oxidation number, then the reaction is a redox reaction. I�m jewish nice plush toys and it�s called sale museum this year.
 Source: homeworklib.com
Source: homeworklib.com
All the processes given above are redox processes as in electrochemical process like extraction of highly reactive metals is a redox reaction. Pb₂²+ + 2br⁻ → pbbr c. Which of the following reactions is a redox reaction?
 Source: zenius.net
Source: zenius.net
Many processes that occur around us are redox reactions. Gain of electron or decrease in oxidation state. The oxidation state of clo is +7.
 Source: numerade.com
Source: numerade.com
Even so in this question they asked which of the following is a redox reaction and scl plus cannot produce an and meticulous case here. The given options are clo, no,so, io. Which of the following are redox reactions?
![Which Of The Following Reactions Is A Redox Reaction? (A).Cuso_(4)+4Nh_(3) To [Cu(Nh_(3))_(4)]So_(4 - Youtube](https://i.ytimg.com/vi/N6TEEBGFyzc/maxresdefault.jpg "Which Of The Following Reactions Is A Redox Reaction? (A).Cuso_(4)+4Nh_(3) To [Cu(Nh_(3))_(4)]So_(4 - Youtube")
Source: youtube.com
Of fe decreases from +3 (in fe 2 0 3) to 0 (in fe) and therefore, fe 2 0 3 acts as an oxidizing agent. Gain of electron or decrease in oxidation state. Of c increases from +2 (in co) to +4 (in c0 2) and therefore, co acts as a reducing agent.
 Source: brainly.in
Source: brainly.in
Of c increases from +2 (in co) to +4 (in c0 2) and therefore, co acts as a reducing agent. Thus, this is a redox reaction. Here, zn0is oxidized to zn+2and ag+1 is reduced to ag0.
 Source: toppr.com
Source: toppr.com
Thus, this is a redox reaction. Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of rules formulated on the basis that electron pair in a covalent bond belongs entirely to more electronegative elements. O is an example of which type of reaction?
Also Read :