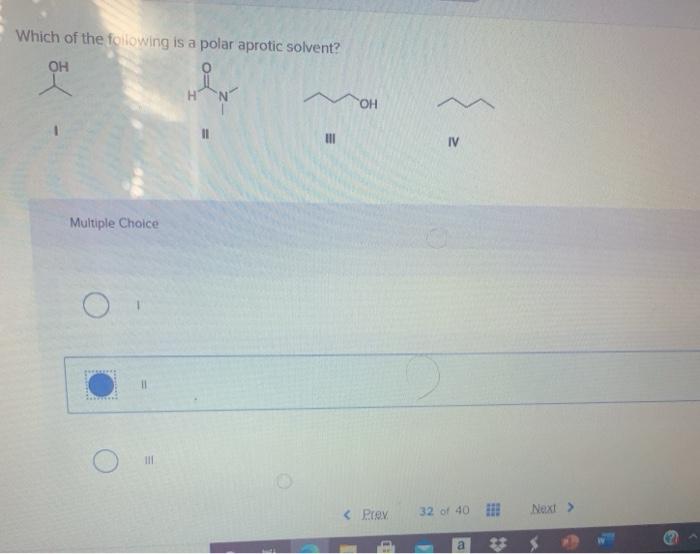
Which of the following is a polar aprotic solvent? Ii i iv iii rank the following ions in order of increasing nucleophlicity in polar protic solvents.
Which Of The Following Is A Polar Aprotic Solvent. Typical examples of polar aprotic solvents include acetone, dmso, dmf, thf, ch 2 cl 2. I⁻ is a better nucleophile than f⁻ in polar protic solvents. Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are not capable of hydrogen bonding. A protic solvent has an h atom bound to o or n.
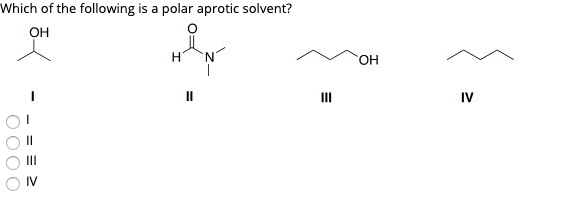 Solved Which Of The Following Is A Polar Aprotic Solvent? Oh | Chegg.com From chegg.com
Solved Which Of The Following Is A Polar Aprotic Solvent? Oh | Chegg.com From chegg.com
Related Post Solved Which Of The Following Is A Polar Aprotic Solvent? Oh | Chegg.com :
11 rows polar aprotic solvents. What is a polar aprotic solvent? Iv<iii<ii<i i<ii<iv<iii i<ii<iii<iv iv<iii<i<ii rank the alkyl halides in order of decreasing s_n^2 reactivity, putting the most reactivity first. If useful please approve it.
Which of the following is a polar aprotic solvent?
Is dmso a good nucleophile? Some commonly used polar aprotic solvents are acetone, dmf, acetonitrile, dmso, etc. Which of the following is a polar aprotic solvent? What is a polar aprotic solvent? In this case nucleophilicity mirrors basicity. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water.
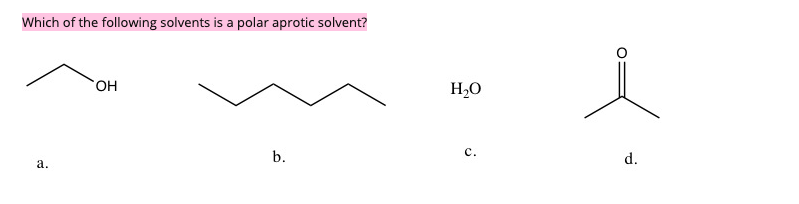 Source: chegg.com
Source: chegg.com
What is a polar aprotic solvent? So, dimethylformamide is a polar aprotic solvent. Polar protic solvents have high dielectric constants and high polarity.
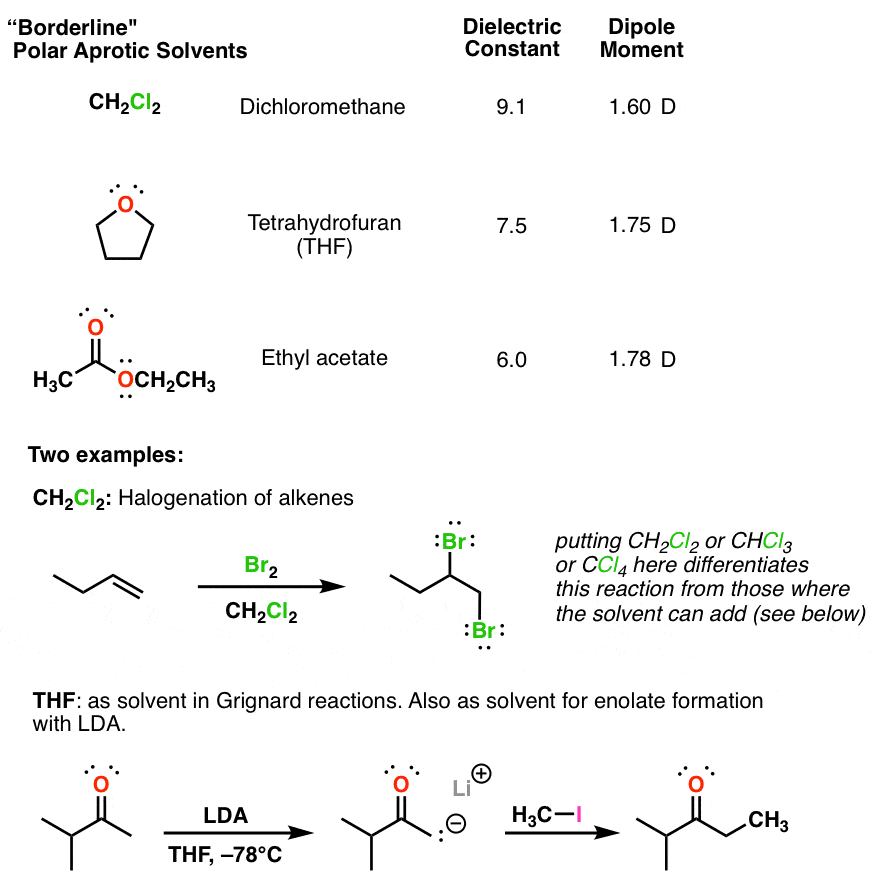 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Reaction is carried out in the presence of polar aprotic solvent. Starting with the last nucleophilic ion. They solvate cations and anions effectively.
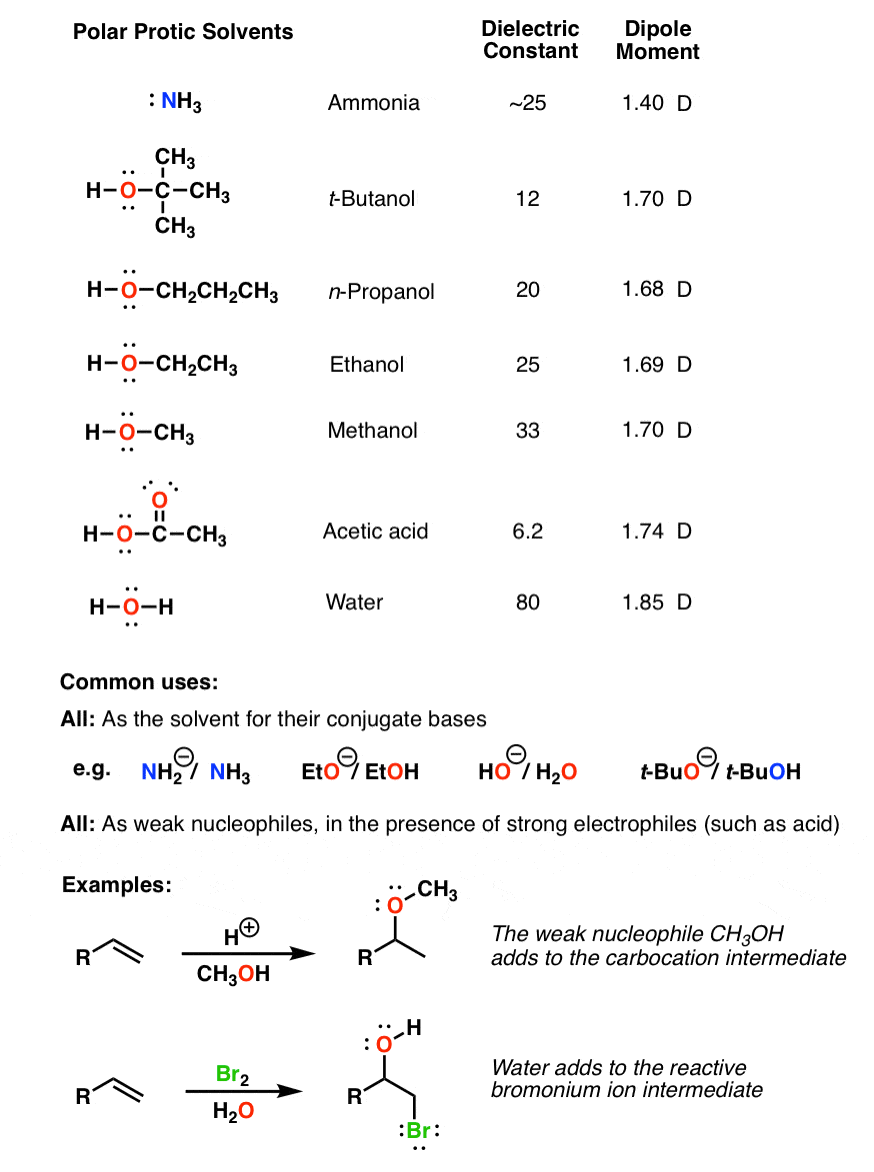 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Complete step by step answer we have seen that various solvents are used in chemical reactions and need to be chosen properly. In this case nucleophilicity mirrors basicity. This mechanism requires a weak base and poor nucleophile.
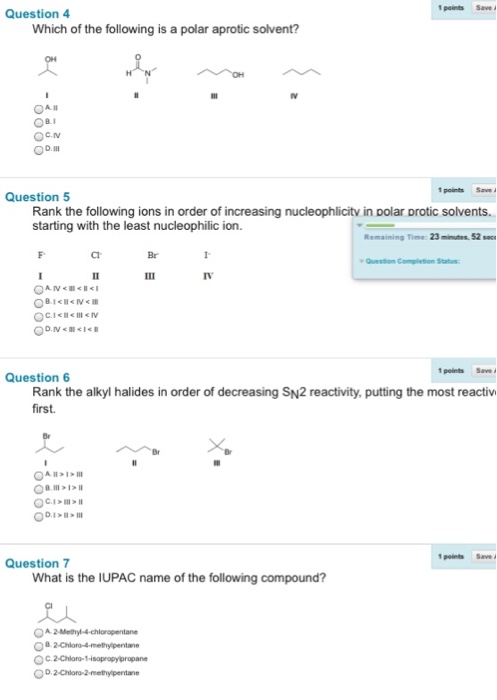
11 rows polar aprotic solvents. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. As marked in the quesiton, the answer choice is e.
 Source: chegg.com
Source: chegg.com
Polar aprotic solvents are those solvents in which we have dipole moment but there is no hydrogen atom attached to electronegative atoms such as oxygen or nitrogen. Complete step by step answer we have seen that various solvents are used in chemical reactions and need to be chosen properly. Which of the following is a polar aprotic solvent?
 Source: quizlet.com
Source: quizlet.com
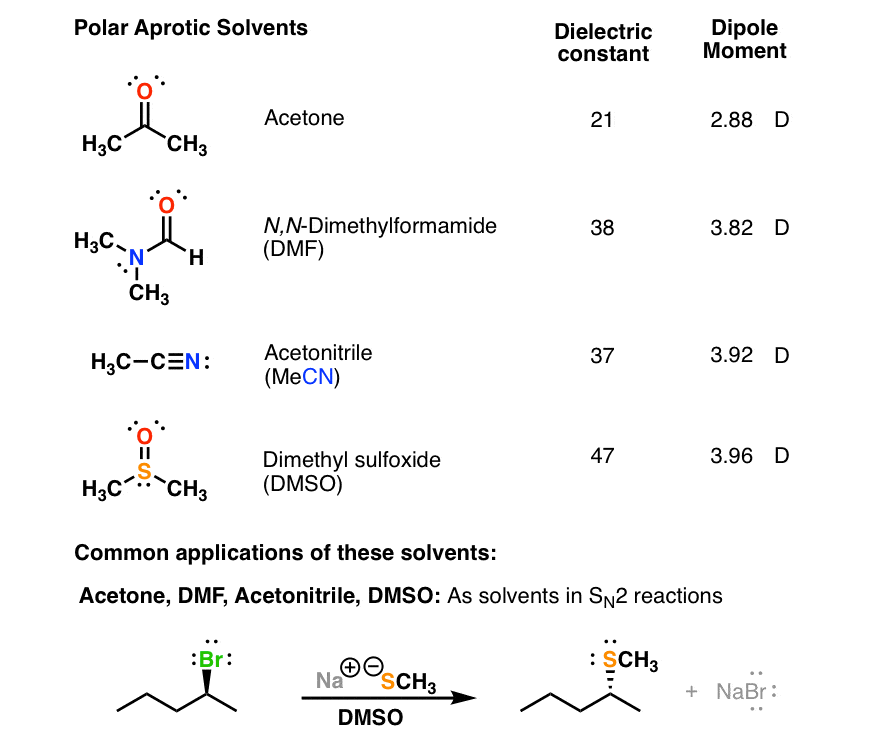
The values for acetone are µ = 2.88 d and ε = 21. Polar protic solvents are water, ethanol, methanol, ammonia, acetic acid, and others. Which of the following is a polar aprotic solvent?
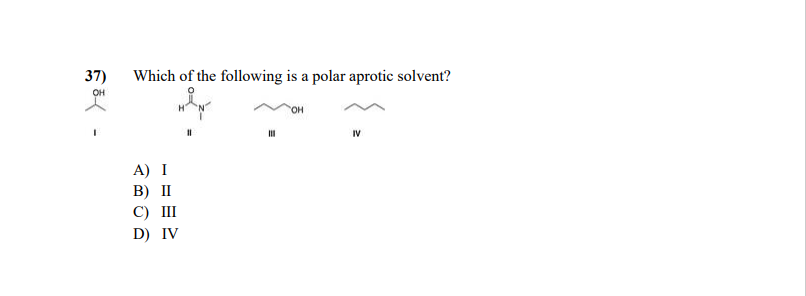
Which one of the following is a polar aprotic solvent? A number of polar aprotic solvents have come into wide use by chemists because they are espcially useful in. For this reason, it becomes important that we should have some basic.
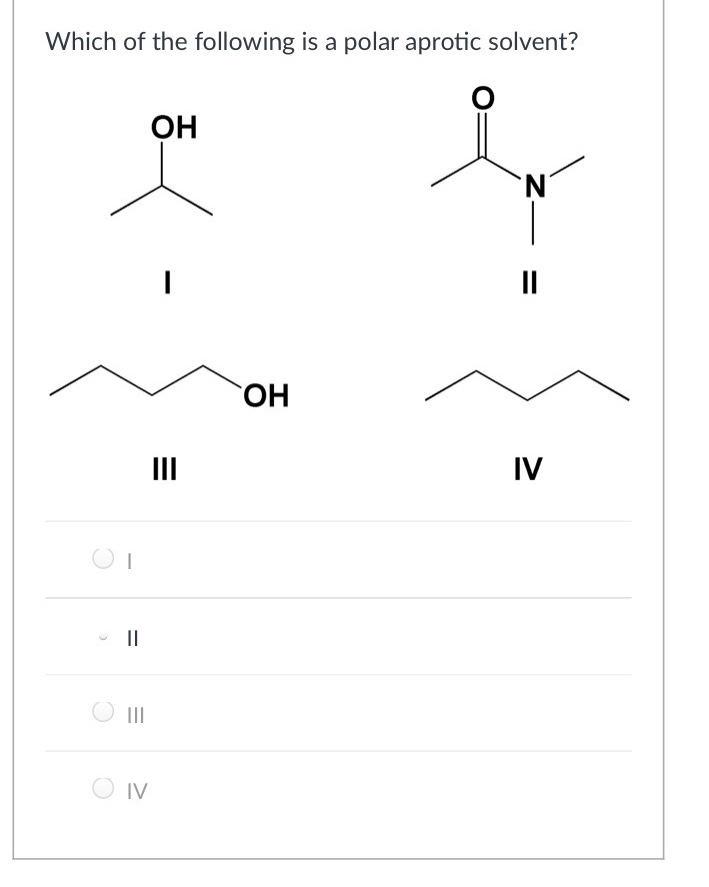 Source: chegg.com
Source: chegg.com
Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom, and they are not capable of hydrogen bonding. Iv<iii<ii<i i<ii<iv<iii i<ii<iii<iv iv<iii<i<ii rank the alkyl halides in order of decreasing s_n^2 reactivity, putting the most reactivity first. So acetone is a polar solvent.
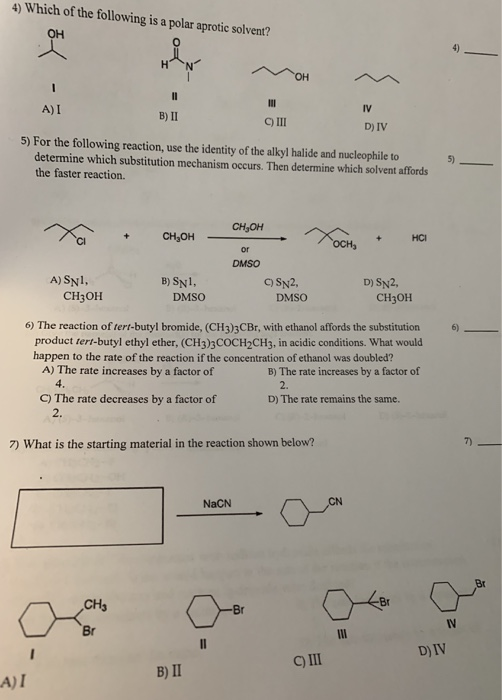 Source: chegg.com
Source: chegg.com
A number of polar aprotic solvents have come into wide use by chemists because they are espcially useful in. Ii i iv iii rank the following ions in order of increasing nucleophlicity in polar protic solvents. Dimethyl sulfoxide (dmso) is an organosulfur compound with the formula (ch 3) 2 so.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
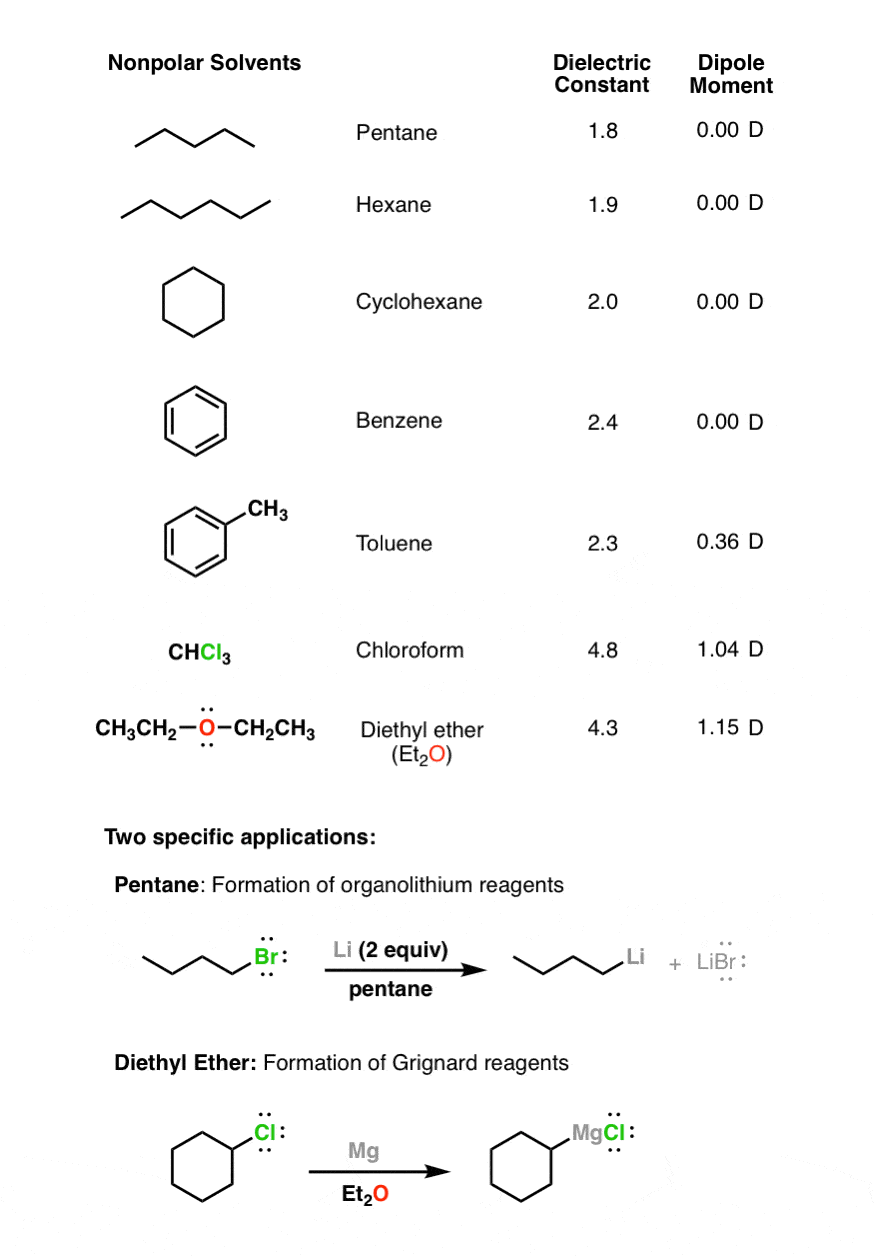
In chemistry, polarity refers to the way in which atoms bond with each other. A solvent is polar if it has a dipole moment greater than 1.6 d and a dielectric constant greater than 5. In chemistry, polarity refers to the way in which atoms bond with each other.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Polar aprotic solvents are those solvents in which we have dipole moment but there is no hydrogen atom attached to electronegative atoms such as oxygen or nitrogen. Which of the following is a polar aprotic solvent? Polar aprotic solvents are those solvents in which we have dipole moment but there is no hydrogen atom attached to electronegative atoms such as oxygen or nitrogen.
 Source: pinterest.com
Source: pinterest.com
Ii i iv iii rank the following ions in order of increasing nucleophlicity in polar protic solvents. All protic solvents are prone to proton reduction to yield hydrogen gas and are only used for reductive electrochemistry. Ii i iv iii rank the following ions in order of increasing nucleophlicity in polar protic solvents.
A polar protic solvent will stabilize this carbocation. If useful please approve it. This mechanism works with a strong base.
 Source: clutchprep.com
Source: clutchprep.com
A polar molecule arises when one of the atoms exerts a stronger attractive force on the electrons in the bond. Dimethyl sulfoxide (dmso) is an organosulfur compound with the formula (ch 3) 2 so. Reaction is carried out in the presence of polar aprotic solvent.
 Source: chemistrysteps.com
Source: chemistrysteps.com
Solvents that are chemically polar in nature and are not capable of hydrogen bonding (implying that a hydrogen atom directly linked with an electronegative atom is not found) are referred to as polar aprotic solvents. Nucleophilicity parallels basicity and the stronger base is the stronger nucleophile. What is a polar aprotic solvent?
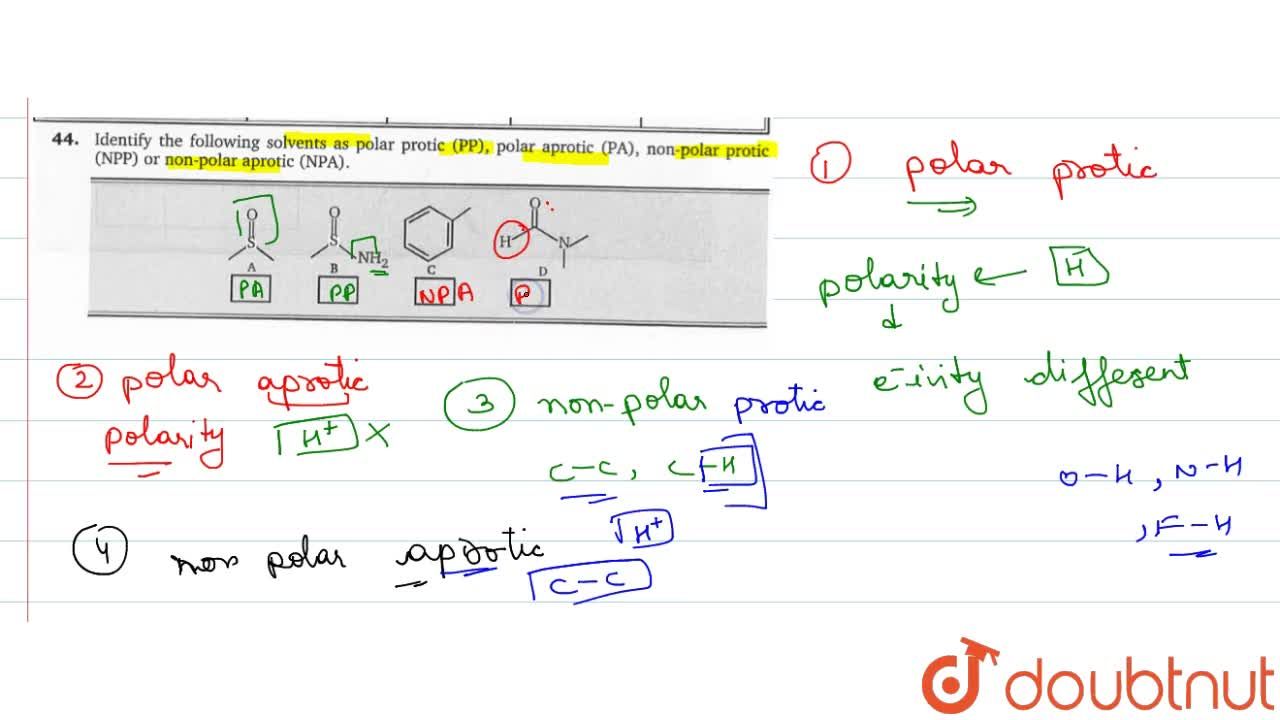 Source: doubtnut.com
Source: doubtnut.com
Polar aprotic solvents contain no hydrogen atoms connected directly to an electronegative atom and they are not capable of hydrogen bonding. They solvate cations and anions effectively. Which of the following is a polar aprotic solvent?
 Source: chegg.com
Source: chegg.com
The solvent for these reactions is polar aprotic. In chemistry, polarity refers to the way in which atoms bond with each other. This mechanism works with a strong base.
 Source: chemistryscore.com
Source: chemistryscore.com
In this case nucleophilicity mirrors basicity. Acetone is a polar aprotic solvent. In this case nucleophilicity mirrors basicity.
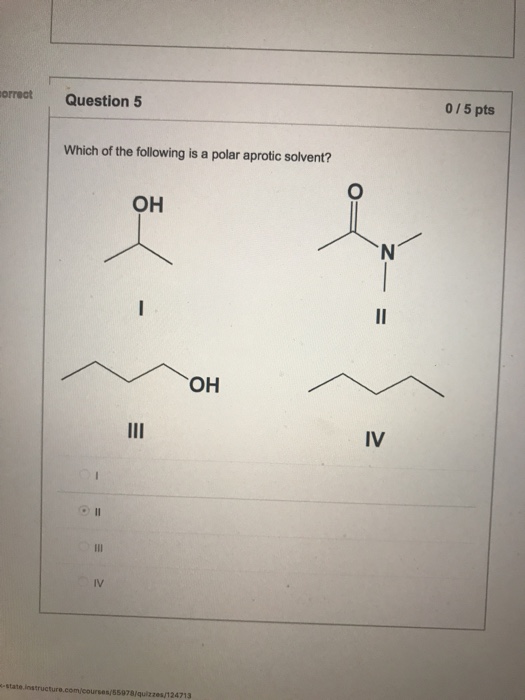
Dimethyl sulfoxide (dmso) is an organosulfur compound with the formula (ch 3) 2 so. Which one of the following is a polar aprotic solvent? Polar aprotic solvents do not contain acidic hydrogen.
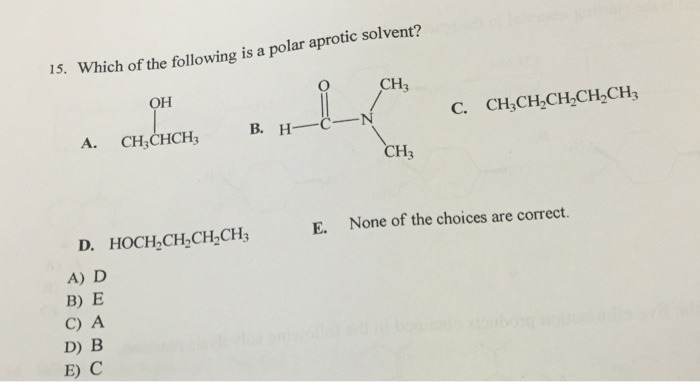
Polar protic solvents are water, ethanol, methanol, ammonia, acetic acid, and others. The solvent for these reactions is polar aprotic. So, dimethylformamide is a polar aprotic solvent.
Also Read :





