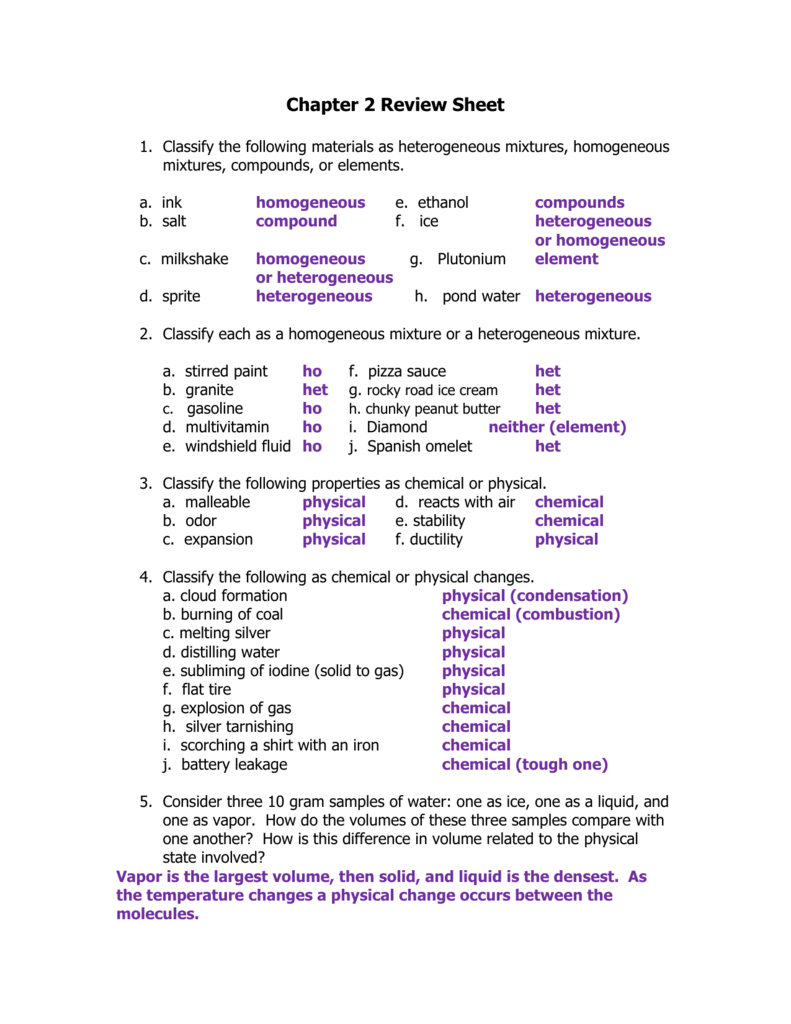
The size of particles is not the same inhomogeneous mixture. The question contains content related to chemistry and science.
Which Of The Following Is A Heterogeneous Mixture. Which out of the following is a heterogeneous mixture? A heterogeneous mixture consists of visibly different phases or substances. Mixing two solids, without melting them together, typically results in a heterogeneous mixture. Gamma rays can cause dna errors, cell death, and lead to the deadly acute radiation syndrome.
 Homogeneous Mixture Examples In Nature From pdfprof.com
Homogeneous Mixture Examples In Nature From pdfprof.com
Related Post Homogeneous Mixture Examples In Nature :
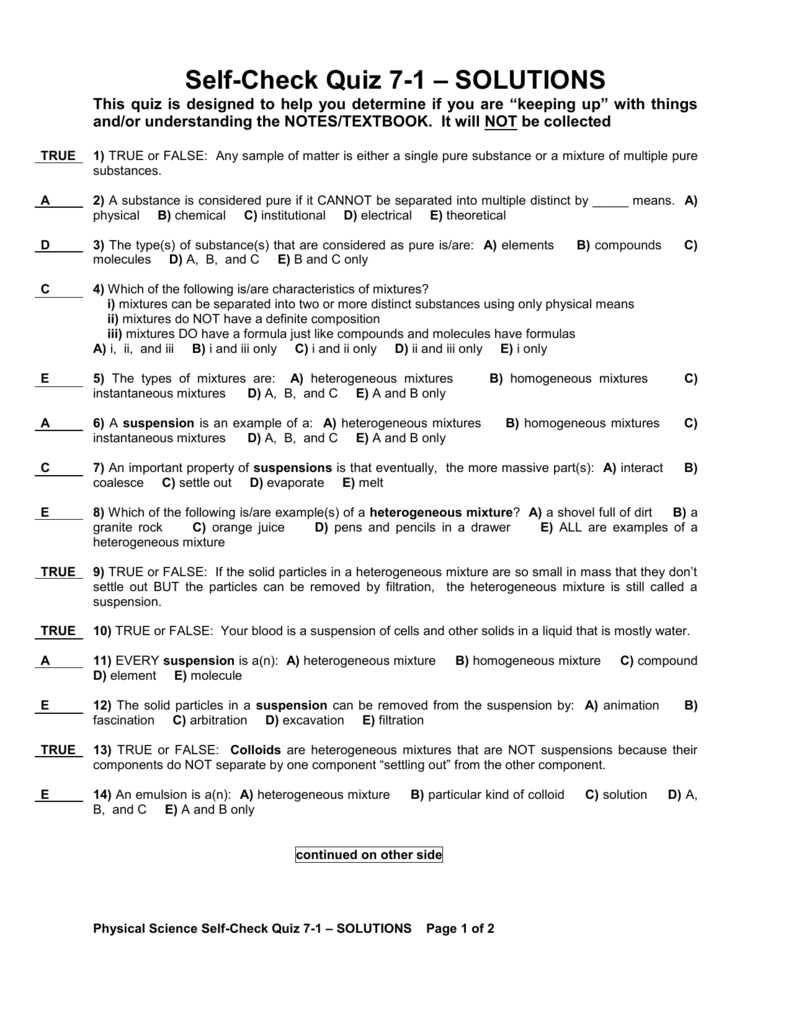
By contrast, a mixture that is uniform in composition is a homogeneous mixture. 3 in 1 coffee dissolved in hot water. A a heterogeneous mixture is a. A sample that contains atoms of only one element.
Chocolate chip cookies are a heterogeneous mixture.
It is the mixture of two or more substances in which constituent particles have homogeneity that means the size of particles is the same. For example, in a solution of salt and water salt cannot be seen. See full list on examples. This 15 words question was answered by heather l. Select a heterogeneous mixture out of the following a air b nacl in water c emulsions d alloy what is the nuclear notation for an element with 26 protons and 30 neutrons. (a) air (b) brass (c) iodised table salt (d) steel.
 Source: chegg.com
Source: chegg.com
Your gift provides uca students with scholarships programs invaluable learning opportunities and. There are two or more phases of a heterogeneous mixture. It is the mixture of two or more substances in which constituent particles have homogeneity that means the size of particles is the same.

Which out of the following is a heterogeneous mixture. A sample that contains atoms of only one element. From the list of given substances:
 Source: pdfprof.com
Source: pdfprof.com
Which of the following is true about heterogeneous mixtures. Concrete is a heterogeneous mixture of an aggregate: Select a heterogeneous mixture out of the following a air b nacl in water c emulsions d alloy what is the nuclear notation for an element with 26 protons and 30 neutrons.
 Source: thoughtco.com
Source: thoughtco.com
Salt and sugar dissolved in water. For example, in a solution of salt and water salt cannot be seen. Since its upload, it has received 228 views.
 Source: clutchprep.com
Source: clutchprep.com
Which of the following islare true about heterogenous mixtures a an. Steel is an alloy of iron and carbon with fixed ratios. Magnesium oxide is not heterogeneous mixture infact it is a pure compound because it contains only magnesium and oxygen atoms.
 Source: slideplayer.com
Source: slideplayer.com
Here are some of the examples of heterogeneous mixtures: A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. From the list of given substances:
 Source: brainly.com
Source: brainly.com
Which of the following is a heterogeneous mixture brainly ph. It is a mixture that has uniform composition throughout. Thus the salad mix is a heterogeneous mixture because it have two or more phases.
 Source: chegg.com
Source: chegg.com
For example, in a solution of salt and water salt cannot be seen. Here are some of the examples of heterogeneous mixtures: 4g of a solute is dissolved in a 40 g of water to form a saturated solution at 25 c 25 c.
 Source: chegg.com
Source: chegg.com
It is the mixture of two or more substances in which constituent particles have homogeneity that means the size of particles is the same. Sugar and sand form a heterogeneous mixture. A heterogeneous mixture is composed of components that are not uniform and have different properties.
 Source: studylib.net
Source: studylib.net
Sugar can dissolve in water completely and form the solution. Water is a homogeneous mixture of nitrogen, oxygen and smaller amounts of other compounds in the gaseous materials. | snapsolve which out of the following is a heterogeneous mixture?( )a.
 Source: expii.com
Source: expii.com
From the list of given substances: 4g of a solute is dissolved in a 40 g of water to form a saturated solution at 25 c 25 c. The question contains content related to chemistry and science.
 Source: thoughtco.com
Source: thoughtco.com
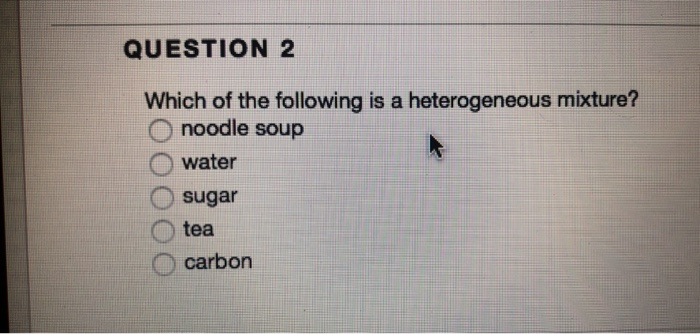
It is the mixture of two or more substances in which constituent particles have homogeneity that means the size of particles is the same. Which of the following is a heterogeneous mixture? Noodle soup is a heterogeneous mixture due to the following reasons:

A a heterogeneous mixture is a. It is made up of two substances that are not miscible in each other. Chocolate chip cookies are a heterogeneous mixture.
 Source: selftution.com
Source: selftution.com
Stainless steel, a jar of mixed nuts, water in a swimming pool, sugar water Particles cannot be seen with the naked eye. A heterogeneous mixture consists of visibly different phases or substances.
 Source: learnwithdrscott.com
Source: learnwithdrscott.com
Noodle soup is a heterogeneous mixture due to the following reasons: Which of the following mixtures is heterogeneous? Which of the following is true of a heterogeneous mixture to assist you understand the concepts, a mixture is a combination of usually two substances.
 Source: studylib.net
Source: studylib.net
4g of a solute is dissolved in a 40 g of water to form a saturated solution at 25 c 25 c. A heterogeneous mixture is a type of mixture that allows the components to be seen as two or more phases are present. New movie trailers were excited about.

The question contains content related to chemistry and science. Which of the following is true about heterogeneous mixtures. Ice cubes in cola form a heterogeneous mixture.
 Source: slideplayer.com
Source: slideplayer.com
4g of a solute is dissolved in a 40 g of water to form a saturated solution at 25 c 25 c. Vegetable salad pinakbet cereals and milk rocks and sand meat stew for more information about homogeneous and heterogeneous mixtures, please visit the link below: Jelly is a mixture of fruit juice and sugar and it is a heterogeneous mixture.
 Source: examples.yourdictionary.com
Source: examples.yourdictionary.com
Therefore dusty air is a heterogeneous mixture just as smoke and fog. See full list on examples. While other three are all heterogeneous mixture.
 Source: brainly.com
Source: brainly.com
It is the mixture of two or more substances in which constituent particles have homogeneity that means the size of particles is the same. Which of the following islare true about heterogenous mixtures a an. For example, in a solution of salt and water salt cannot be seen.
Also Read :





