D) the conjugate base of a very weak acid is stronger than the conjugate base of a strong acid. Conjugate acids and conjugate bases are the acids and bases that lose or gain protons.
Which Of The Following Is A Conjugate Acid Base Pair. So the anwer could only be the first pair: Some common examples for formation conjugate bases. Conjugate acid base pairs formed from each other mutually by the gain or loss of protons. The weaker its conjugate base.
 Answered: 58. Which Of The Following Is Not A… | Bartleby From bartleby.com
Answered: 58. Which Of The Following Is Not A… | Bartleby From bartleby.com
Related Post Answered: 58. Which Of The Following Is Not A… | Bartleby :
The term conjugate comes from the latin stems meaning joined together and refers to things that are joined, particularly in pairs, such as brnsted acids and bases. The stronger acid and weaker base form one conjugate pair and the stronger base and weaker acid form another pair. The acid is the compound that contains the additional h⁺ the conjugate base of h₂so₃ is hso₃⁻. Three doses of the hepatitis b vaccine (at birth and 1 and 6 months of age);
Answer (4) h20+ h2o +h* conjugate acid base.
The child has previously received the following vaccines: Similarly, the stronger the base, the weaker is its conjugate acid. So the anwer could only be the first pair: Conjugate acid base pairs formed from each other mutually by the gain or loss of protons. The child has previously received the following vaccines: The term conjugate comes from the latin stems meaning joined together and refers to things that are joined, particularly in pairs, such as bronsted acids and bases.
 Source: youtube.com
Source: youtube.com
True the salt that forms due to neutralization of phosphoric acid by calcium hydroxide has the formula ca3p2. Some common examples for formation conjugate bases. Three doses of the hepatitis b vaccine (at birth and 1 and 6 months of age);
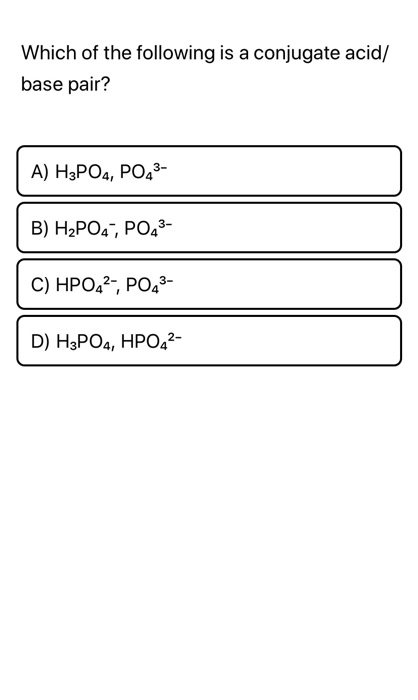 Source: chegg.com
Source: chegg.com
The stronger acid and weaker base form one conjugate pair and the stronger base and weaker acid form another pair. A conjugate acid comprises one more h atom and +charge than the base that formed it. The term conjugate comes from the latin stems meaning joined together and refers to things that are joined, particularly in pairs, such as bronsted acids and bases.
 Source: clutchprep.com
Source: clutchprep.com
Similarly, water is the conjugate base of hydronium ion and hydronium ion is the conjugate acid of water. Conjugate acids and conjugate bases are the acids and bases that lose or gain protons. The following three equations represent equilibria that lie far to the right.

The stronger acid and weaker base form one conjugate pair and the stronger base and weaker acid form another pair. Hno 3 and hno 2 e. The stronger acid and weaker base form one conjugate pair and the stronger base and weaker acid form another pair.
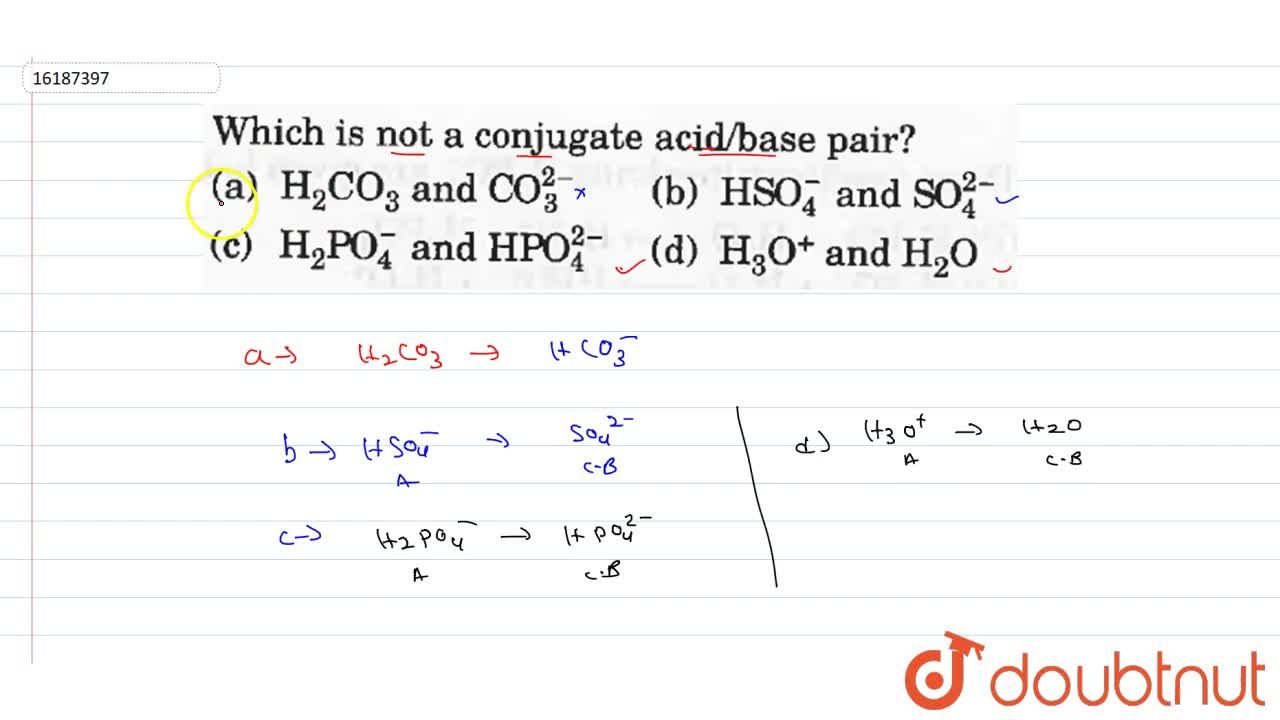 Source: doubtnut.com
Source: doubtnut.com
A conjugate acid is the species that. Similarly, the stronger the base, the weaker is its conjugate acid. A conjugate acid comprises one more h atom and +charge than the base that formed it.
 Source: toppr.com
Source: toppr.com
The stronger the acid, the weaker is its conjugate base. The acid is the compound that contains the additional h⁺ the conjugate base of h₂so₃ is hso₃⁻. The term conjugate comes from the latin stems meaning joined together and refers to things that are joined, particularly in pairs, such as brnsted acids and bases.
 Source: clutchprep.com
Source: clutchprep.com
O) is dissolved in water, the solution turns basic from the reaction of the oxide ion (o. The stronger the acid, _____ a. Nh4+ is the conjugate acid to the base nh3, because nh3 gained a hydrogen ion to form nh4+.the conjugate base of an acid is formed when the acid donates a proton.
 Source: chegg.com
Source: chegg.com
True the salt that forms due to neutralization of phosphoric acid by calcium hydroxide has the formula ca3p2. The term conjugate comes from the latin stems meaning joined together and refers to things that are joined, particularly in pairs, such as bronsted acids and bases. Answer the following in brief :
![Complete The Following Table Of Conjugate Acid/Base Pairs. Complete The Following Table Of Conjugate Acid/Base Pairs. [{Image Src=�Base1709148556484445437.Jpg� Alt=�Base� Caption=��}] Source: study.com
Similarly, water is the conjugate base of hydronium ion and hydronium ion is the conjugate acid of water. The following three equations represent equilibria that lie far to the right. A conjugate acid is the species that.
 Source: targetbatch.com
Source: targetbatch.com
Three doses of the diphtheria/tetanus/acellular pertussis (dtap) vaccine (at 2, 4. The stronger the acid, the weaker is its conjugate base. So, nh 4 + is the strong conjugate acid of nh3.
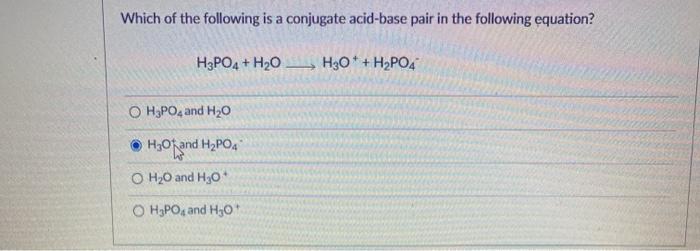
The term conjugate comes from the latin stems meaning joined together and refers to things that are joined, particularly in pairs, such as brnsted acids and bases. Answer the following in brief : The conjugate base of nh₄⁺ is nh₃.
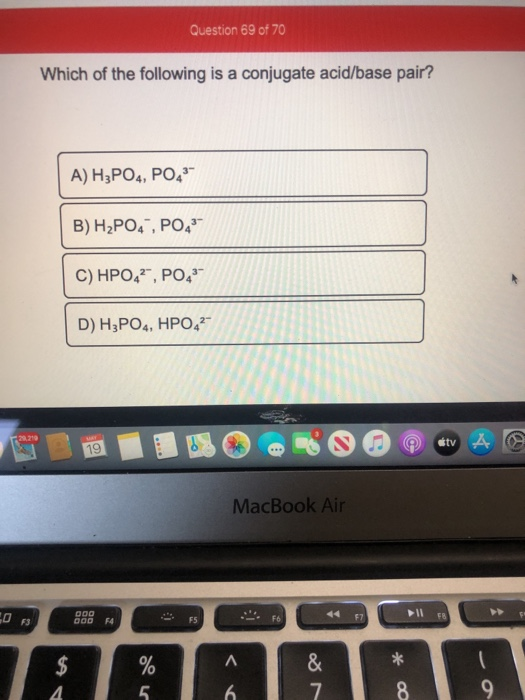 Source: chegg.com
Source: chegg.com
Some common examples for formation conjugate bases. Nh4+ is the conjugate acid to the base nh3, because nh3 gained a hydrogen ion to form nh4+.the conjugate base of an acid is formed when the acid donates a proton. The less concentrated the conjugate base.
![Solved] For The Reaction Shown Below, Which Of The Following Is A Conjugate Acid-Base Pair? | Course Hero](https://www.coursehero.com/qa/attachment/3395079/ “Solved] For The Reaction Shown Below, Which Of The Following Is A Conjugate Acid-Base Pair? | Course Hero”) Source: coursehero.com
The equilibrium reaction involves two acids and two bases. The stronger its conjugate base. Which of the following acids will have the strongest conjugate base?
 Source: numerade.com
Source: numerade.com
Similarly, the stronger the base, the weaker is its conjugate acid. Three doses of the hepatitis b vaccine (at birth and 1 and 6 months of age); Similarly, water is the conjugate base of hydronium ion and hydronium ion is the conjugate acid of water.
 Source: chegg.com
Source: chegg.com
Three doses of the diphtheria/tetanus/acellular pertussis (dtap) vaccine (at 2, 4. We also have water and hydronium, which are also related by that one h plus. Conjugate acid base pairs formed from each other mutually by the gain or loss of protons.
 Source: slidetodoc.com
Source: slidetodoc.com
The conjugate acid of c₂h₃o₂⁻ is hc₂h₃o₂ and conjugate base of h₃o⁺ is h₂o, not oh⁻. So, nh 4 + is the strong conjugate acid of nh3. The conjugate acid of c₂h₃o₂⁻ is hc₂h₃o₂ and conjugate base of h₃o⁺ is h₂o, not oh⁻.

O) is dissolved in water, the solution turns basic from the reaction of the oxide ion (o. The conjugate base of nh₄⁺ is nh₃. The child has previously received the following vaccines:
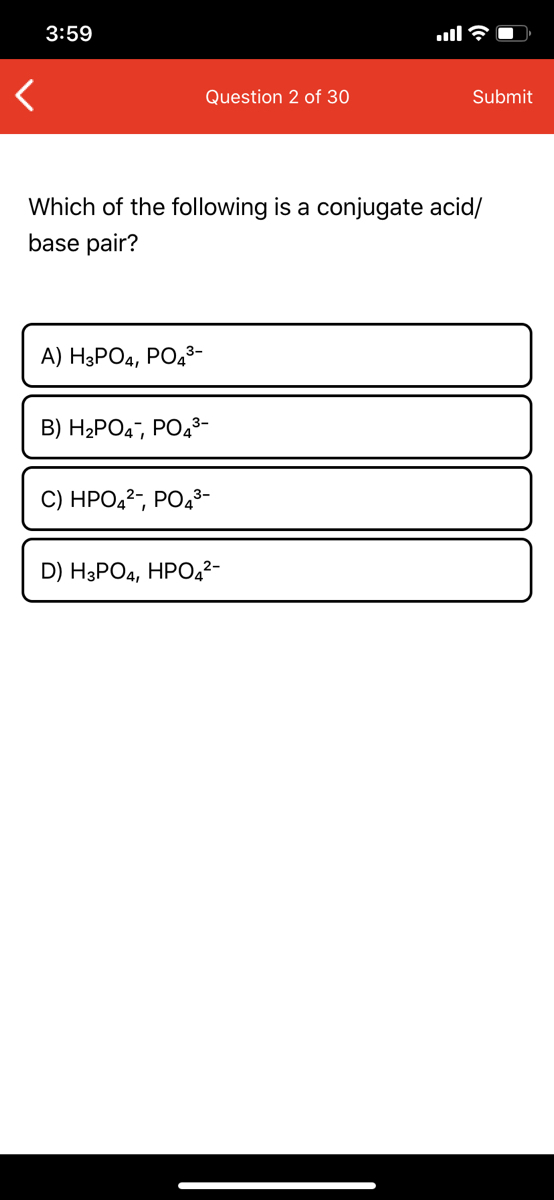 Source: bartleby.com
Source: bartleby.com
Imagine a generic acid, ha. The following three equations represent equilibria that lie far to the right. The conjugate base of nh₄⁺ is nh₃.
 Source: bartleby.com
Source: bartleby.com
A conjugate acid comprises one more h atom and +charge than the base that formed it. O) is dissolved in water, the solution turns basic from the reaction of the oxide ion (o. Similarly, water is the conjugate base of hydronium ion and hydronium ion is the conjugate acid of water.
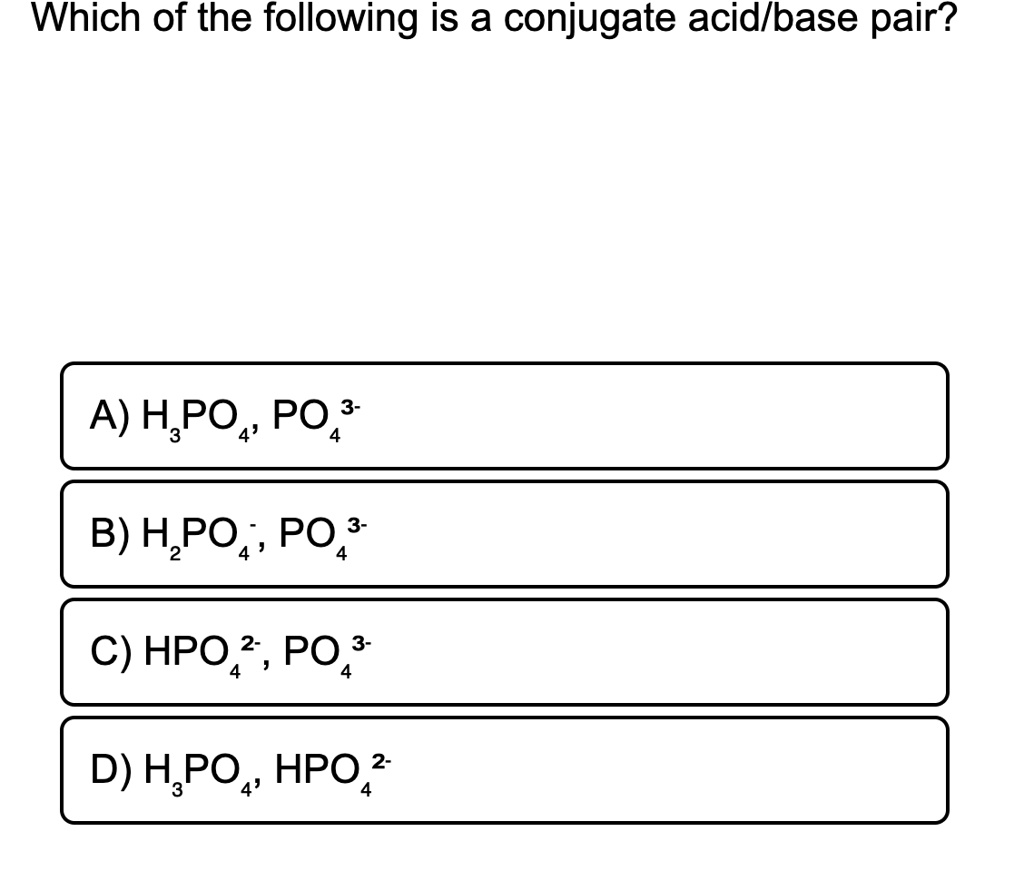 Source: numerade.com
Source: numerade.com
C) a h 2 o molecule may react as an acid by donating a proton. The conjugates will always be listed on the product side of the. For example, the conjugated acid and base pairs of acetic acid are represented.
Also Read :





