Login is required in order to view results and track your progress. The second option is h 2 o (water).
Which Of The Following Is A Bronsted Lowry Acid. Hclo 2 e) h 2 so 4; It very well could accept a hydrogen ion. Bronsted acid is an h+ donor, bronsted base is an h+ acceptor. Usually bronsted acids have an h bonded to a halogen or an oxygen.
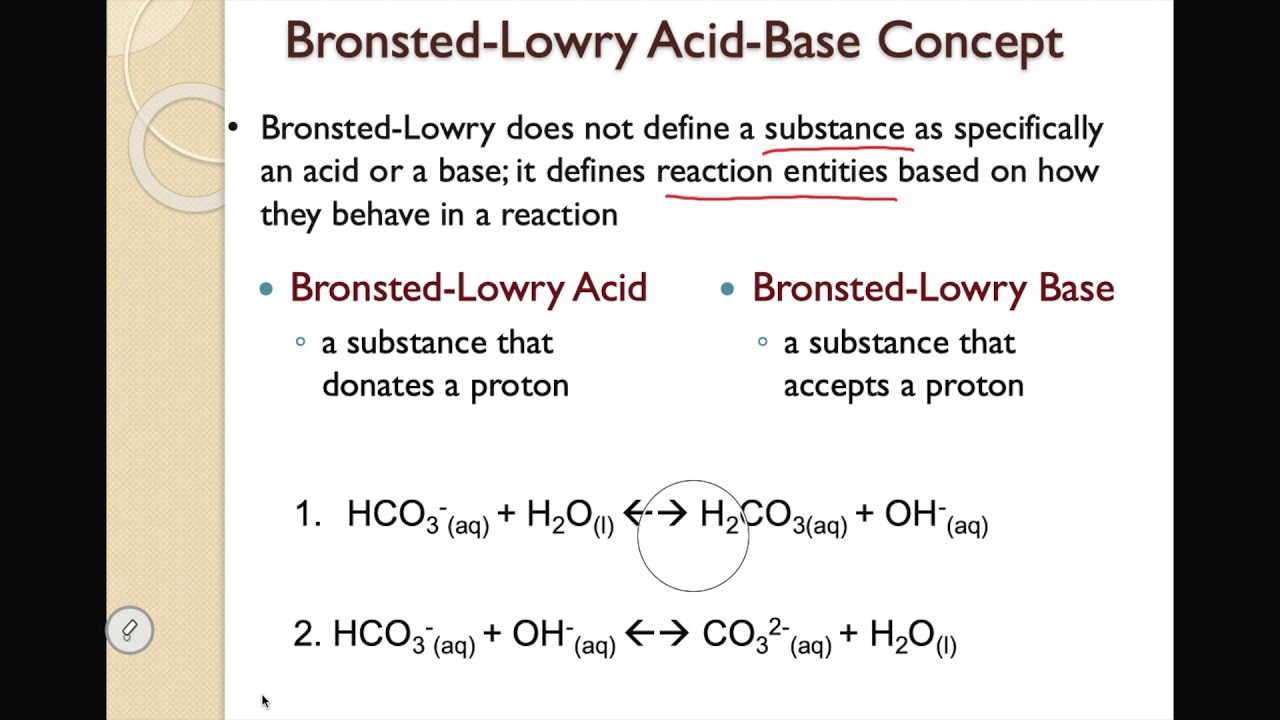 Bronsted-Lowry Acid-Base Concept - Youtube From youtube.com
Bronsted-Lowry Acid-Base Concept - Youtube From youtube.com
Related Post Bronsted-Lowry Acid-Base Concept - Youtube :
So it is a bronston lowry base. Its conjugate acid is the ammonium ion. When an acid dissolves in water it donates the h + molecules to h2o and forms h3o +. Hno 2 b) h 2 so 3;
Any species that will donate h+ (protons) in solution, and makes ph lower (i.e hcl) bronsted lowry base:
According to the theory, acid and base react with each other and by an exchange of proton acid, forms its conjugate base and the base forms its. It very well could accept a hydrogen ion. H 2 s + h 2 o ⇆ h 3 o + + s h −. When an acid dissolves in water it donates the h + molecules to h2o and forms h3o +. This last molecule has several lone pairs. Of course, this equilibrium lies to the left, as it does for weakly acidic hydrogen fluoride.
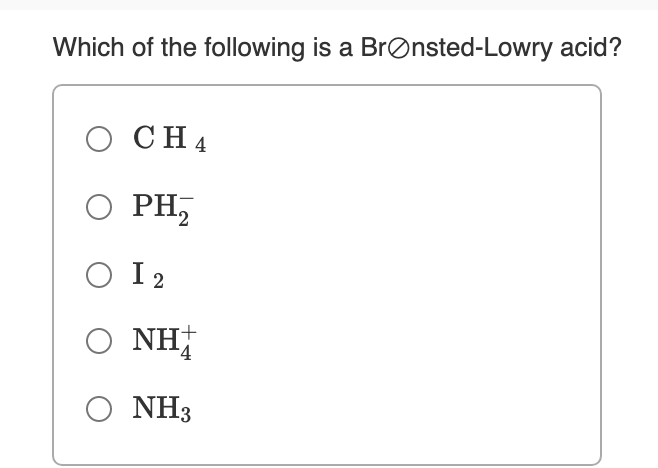 Source: bartleby.com
Source: bartleby.com
Bronsted acid is an h+ donor, bronsted base is an h+ acceptor. The chloride ion is its conjugate base. B) basic which of the following salts, when dissolved in water, produces the solution with the highest ph?
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Out of all the four complex ions, the only one that can donate a proton is the hexa aquairon (iii)ion [fe(h2 o)6 ]3+. Hno 2 b) h 2 so 3; H 2 seo 3 c) hclo 4;
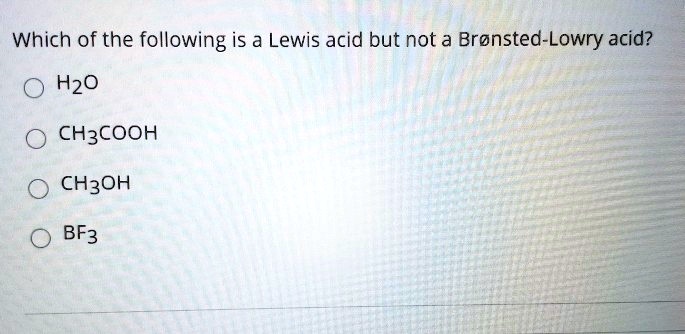 Source: numerade.com
Source: numerade.com
H c o 3 − is the conjugate base of carbonic acid and the conjugate acid of the carbonate ion. (2) both acidic and nonacidic hydrogen atoms may be present in an acid molecule. Which one of the following pairs of acids is incorrectly listed?
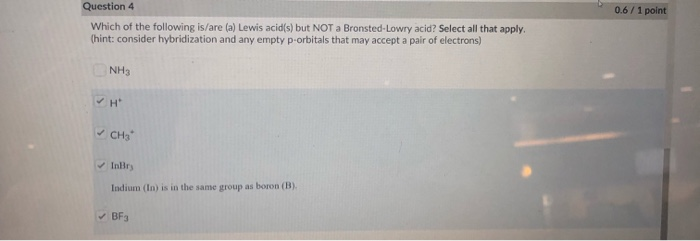 Source: chegg.com
Source: chegg.com
H2o is amphoteric so it can act as both a bronsted acid or base at neutral conditions. H c o 3 − is the conjugate base of carbonic acid and the conjugate acid of the carbonate ion. Hydrobromic acid, hbr, is a strong acid, meaning it practically entirely dissociates in water.
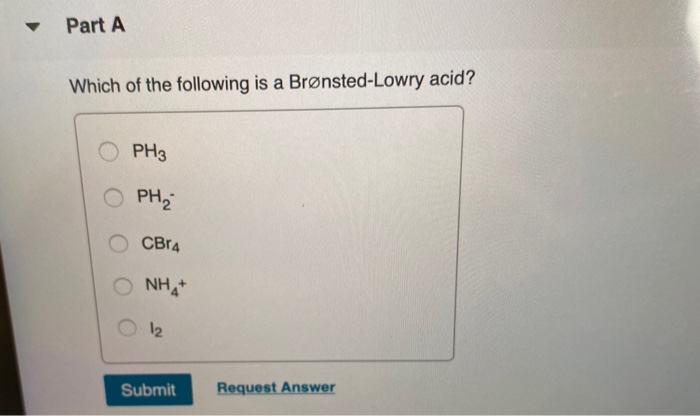 Source: chegg.com
Source: chegg.com
Aslso, the proton, may combine h + of. Out of all the four complex ions, the only one that can donate a proton is the hexa aquairon (iii)ion [fe(h2 o)6 ]3+. Hydrobromic acid, hbr, is a strong acid, meaning it practically entirely dissociates in water.

H 3 o + c. H 2 s + h 2 o ⇆ h 3 o + + s h −. The second option is h 2 o (water).
 Source: chegg.com
Source: chegg.com
Hydrobromic acid, hbr, is a strong acid, meaning it practically entirely dissociates in water. Aslso, the proton, may combine h + of. A bronsted lowry acid is defined simply as a proton donor.

Weaker acid) a) hno 3; Since the question does not specify conditions, we should assume this to be at a neutral condition (ph = 7) which would make nh3 a base. Hence it can act as both acid and base.
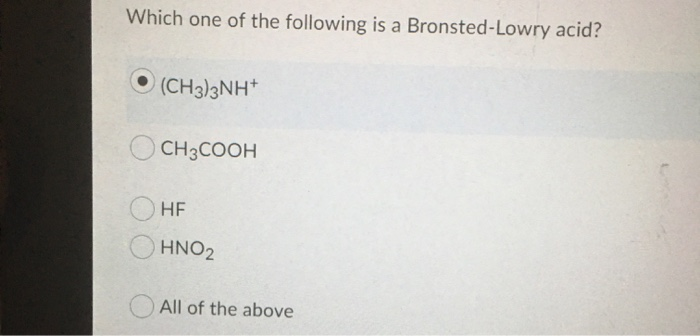 Source: chegg.com
Source: chegg.com
But these species are much too reactive to. B) basic which of the following salts, when dissolved in water, produces the solution with the highest ph? The chloride ion is its conjugate base.
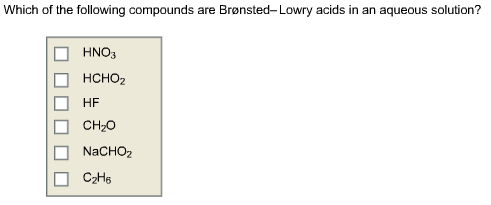 Source: chegg.com
Source: chegg.com
Therefore, h 2 s (hydrogen sulphide) acts as a bronsted acid but not a bronsted base. Weaker acid) a) hno 3; I�m concerned that the complexity of this molecule is what the authors air referring to where it is less.

Weaker acid) a) hno 3; Acids release a proton, or h+, in water. When an acid dissolves in water it donates the h + molecules to h2o and forms h3o +.
 Source: youtube.com
Source: youtube.com
Charge can accept the hydrogen ion. (2) both acidic and nonacidic hydrogen atoms may be present in an acid molecule. It is a bronston lowry base water can accept a hydrogen ion that has two lone pairs here.
 Source: chegg.com
Source: chegg.com
H 3 po 4 d. For the lewis theory, acid is the substance that can gain a pair of electrons, and the base is the substances that can donate the pair of electrons. The chloride ion is its conjugate base.
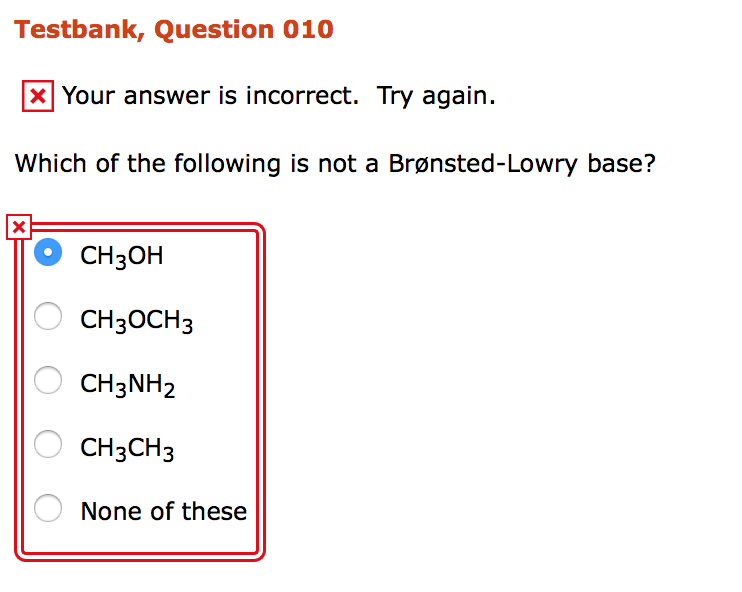 Source: chegg.com
Source: chegg.com
H2o is amphoteric so it can act as both a bronsted acid or base at neutral conditions. Since the question does not specify conditions, we should assume this to be at a neutral condition (ph = 7) which would make nh3 a base. If an equal number of moles of the weak acid hf and the strong base koh are added to water, is the resulting solution acidic, basic, or neutral?
 Source: numerade.com
Source: numerade.com
Weaker acid) a) hno 3; The chloride ion is its conjugate base. H 2 s ⇌ h s − + h +.
 Source: youtube.com
Source: youtube.com
H c o 3 − is the conjugate base of carbonic acid and the conjugate acid of the carbonate ion. Any species that will donate h+ (protons) in solution, and makes ph lower (i.e hcl) bronsted lowry base: H c o 3 − is the conjugate base of carbonic acid and the conjugate acid of the carbonate ion.
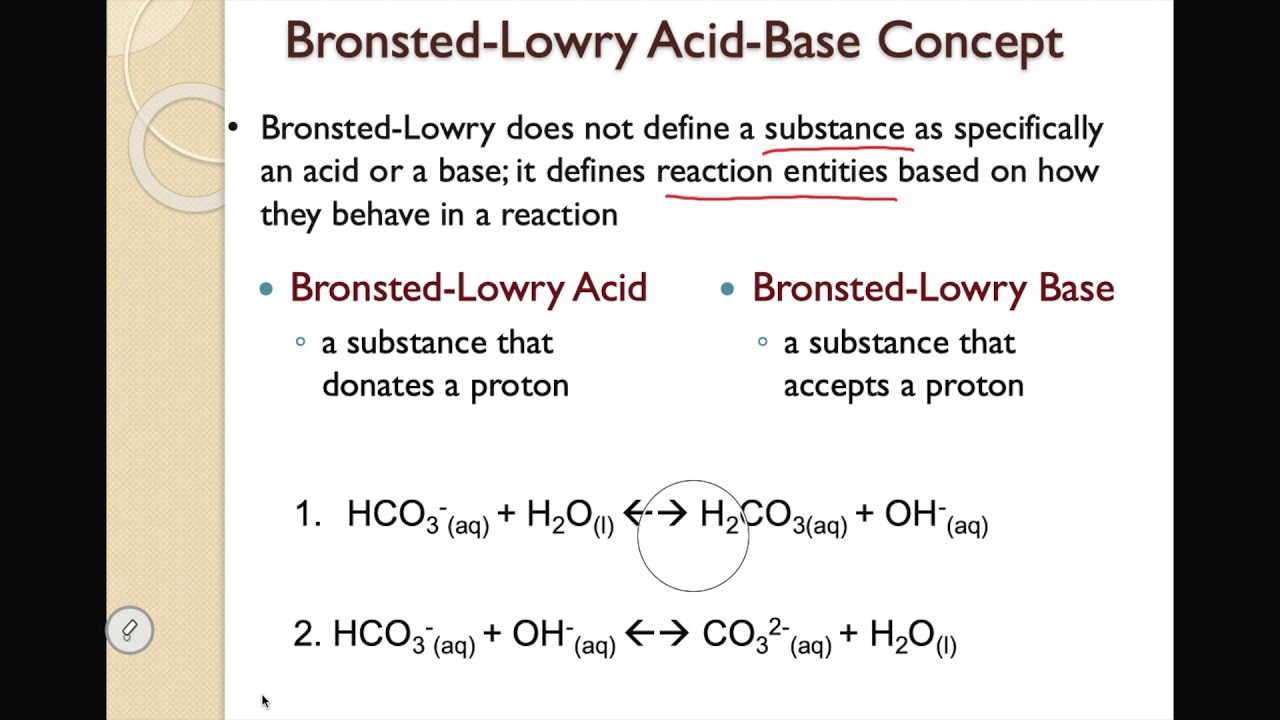 Source: youtube.com
Source: youtube.com
H so− 3 + h 2o ⇌ h 3o+ +so2− 3. A bronsted lowry acid is defined simply as a proton donor. I�m concerned that the complexity of this molecule is what the authors air referring to where it is less.

Its an even stronger acid than hydrochloric acid, and stronger than hbro. Since the question does not specify conditions, we should assume this to be at a neutral condition (ph = 7) which would make nh3 a base. Which one of the following pairs of acids is incorrectly listed?
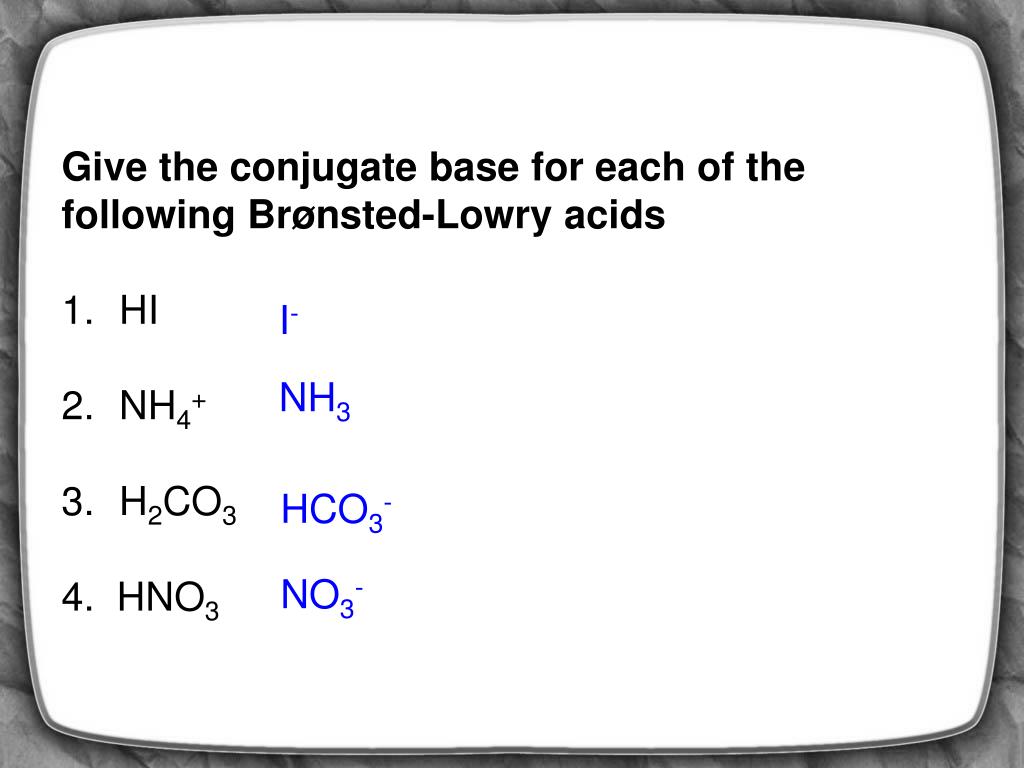 Source: slideserve.com
Source: slideserve.com
The chloride ion is its conjugate base. Usually bronsted acids have an h bonded to a halogen or an oxygen. On the other hand, cl2, can donate no protons to the solvent, and is not classified as an acid.
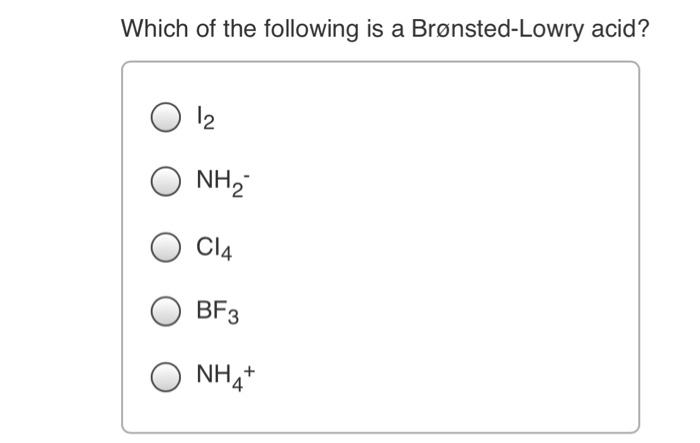 Source: chegg.com
Source: chegg.com
H 2 s + h 2 o ⇆ h 3 o + + s h −. Any species that will donate h+ (protons) in solution, and makes ph lower (i.e hcl) bronsted lowry base: Usually bronsted acids have an h bonded to a halogen or an oxygen.
Also Read :





