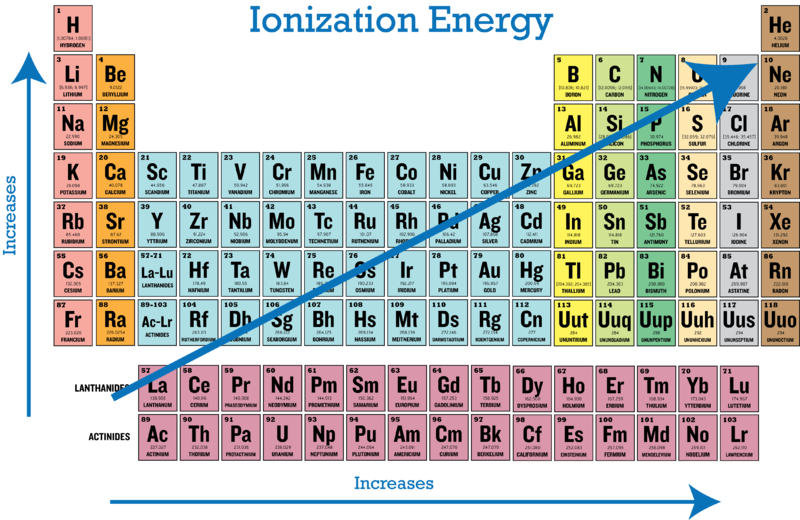
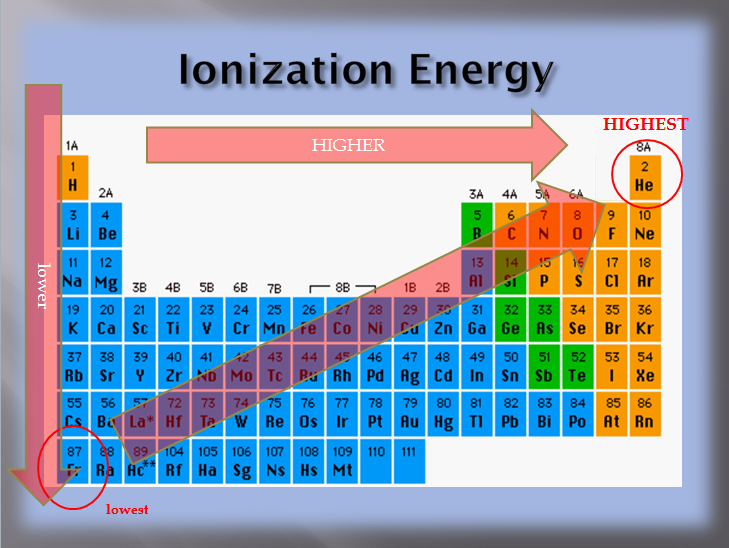
Ionization energy is the minimum amount of energy required to remove the electron from outermost shell (termed as first ionization energy) of an isolated atom. Thus, helium has the largest first ionization energy, while francium has one of the lowest.
Which Of The Following Has The Lowest Ionization Energy. Alkali metalsalkali metals have the lowest first ionisation energy. 1s 22s 22p 63s 1. It is known to us, first ionization energy decreases with. Of protons that acts as an attraction force between protons and electrons) so.
Related Post Chemistry Express: Ions And Ionization Energy — Steemit :
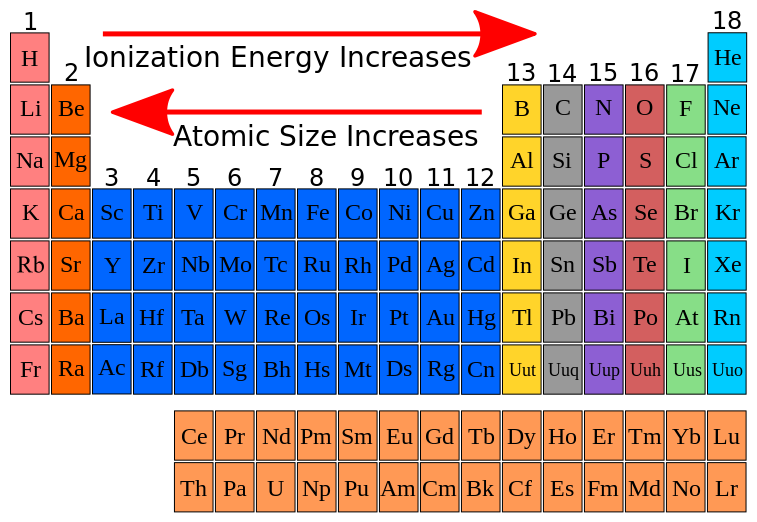
1s 22s 22p 63s 1. Down a group, first ionization energy decreases and atomic radius increases. From this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of. Thus, helium has the largest first ionization energy, while francium has one of the lowest.
1s 22s 22p 63s 1.
So,now arranging above mentioned atoms ( cl,p,ca) you�ll notice that calcium is in 2nd group at 3rd position,phosphorus lies in 5b group at 2nd position and chlorine lies in 7b group at 2nd position.so as calcium is below both atoms so it is larger in. 104 rows to list the elements order by ionization energy, click on the table headers. Thus, helium has the largest first ionization energy, while francium has one of the lowest. Thus, helium has the largest first ionization energy, while francium has one of the lowest. Hence, ionisation energy of lithium ( l i) is lowest in the given elements. But k has only one electron in its outermost energy level of its electronic configuration.
 Source: socratic.org
Source: socratic.org
What is ionization energy in simple words? S is the element of the 3rd period. The first ionization energy varies in a predictable way across the periodic table.
 Source: examples.yourdictionary.com
Source: examples.yourdictionary.com
But k has only one electron in its outermost energy level of its electronic configuration. Kr is an inert gas. From this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of.
 Source: youtube.com
Source: youtube.com
Kr is an inert gas. Thus, helium has the largest first ionization energy, while francium has one of the lowest. What is lowest ionization energy?

Do main group metals have low first ionization energy? Alkali metalsalkali metals have the lowest first ionisation energy. What is lowest ionization energy?
 Source: physicalsciencetext.weebly.com
Source: physicalsciencetext.weebly.com
104 rows to list the elements order by ionization energy, click on the table headers. Which best explains why ionization energy tends to decrease from the top to the bottom of a group? But k has only one electron in its outermost energy level of its electronic configuration.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
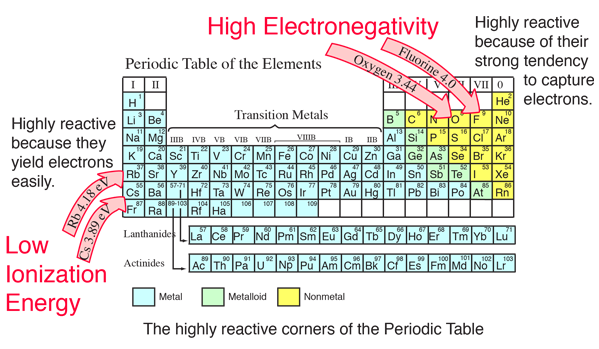
Since removing the second electron from mg gives it the noble gas configuration of 1s22s22p6, which is highly stable, the second ionization energy is only twice larger than the first one. In the periodic table, the minimum ionisation energy is found at alkali metals. Which has low ionization potential?
 Source: socratic.org
Source: socratic.org
Ionization energy value decreases from top to bottom in a group because the shielding effect in atoms increases as we move down a group. Ionization energy, also called ionization potential, in chemistry and physics, the amount of energy required to remove an. Thus, helium has the largest first ionization energy, while francium has one of the lowest.
 Source: hyperphysics.phy-astr.gsu.edu
Source: hyperphysics.phy-astr.gsu.edu
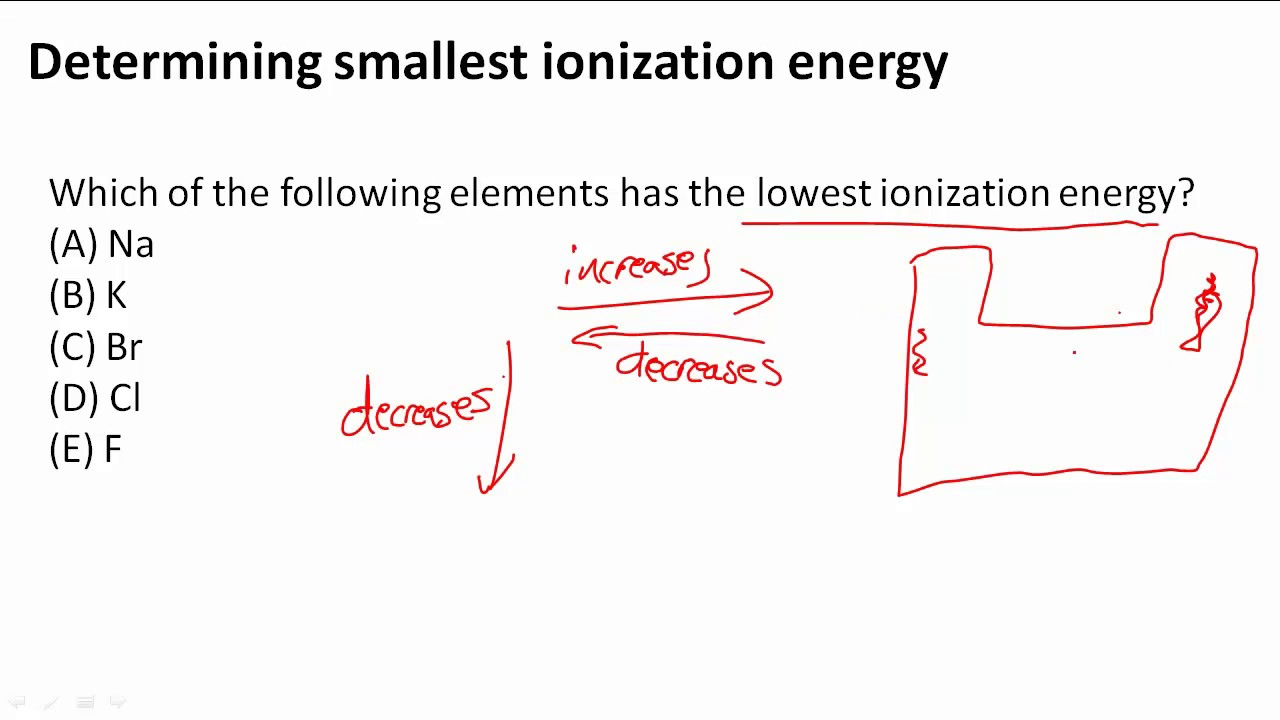
Cesiumfrom this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of helium and neon). Cesiumcesium has the smallest ionization energy since it has the largest amount of energy levels, creating a smaller attractive force making it easier to remove the electron. Ionization energy is the minimum amount of energy required to remove the electron from outermost shell (termed as first ionization energy) of an isolated atom.
 Source: angelo.edu
Source: angelo.edu
From this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of helium and neon). From this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of. Hence sodium (electronic configuration = 1 s 2 2 s 2 2 p 6 3 s 1) has the lowest ionisation energy.
 Source: angelo.edu
Source: angelo.edu
Is larger than the magnesium atom the shielding effect makes it easier to remove the outermost electrons form those atoms which have many electrons. What family has the lowest first ionization energy? So,now arranging above mentioned atoms ( cl,p,ca) you�ll notice that calcium is in 2nd group at 3rd position,phosphorus lies in 5b group at 2nd position and chlorine lies in 7b group at 2nd position.so as calcium is below both atoms so it is larger in.
 Source: youtube.com
Source: youtube.com
From this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of helium and neon). Now in the case of na and mg ,na has lower ionization energy and mg has higher. What is fluorines ionization energy?
 Source: quizlet.com
Source: quizlet.com
Down a group, first ionization energy decreases and atomic radius increases. Compared to the ionization energy of a magnesium atom, the ionization energy of a calcium atom is smaller, this is primarily because the calcium atom: Alkali metalsthe group of elements which have the lowest ionization energy are the alkali metals.
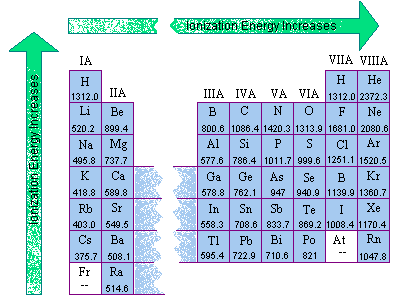 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
Is larger than the magnesium atom the shielding effect makes it easier to remove the outermost electrons form those atoms which have many electrons. If you want to know that of which atom ionization energy is minimum always arrange it according to group and period of. It is known to us, first ionization energy decreases with.
 Source: sciencenotes.org
Source: sciencenotes.org
Ionization energy is the minimum amount of energy required to remove the electron from outermost shell (termed as first ionization energy) of an isolated atom. Ionization energy is the minimum amount of energy required to remove the electron from outermost shell (termed as first ionization energy) of an isolated atom. Which one of the following has lowest first ionization energy?
 Source: en.wikipedia.org
Source: en.wikipedia.org
Ionization energy, also called ionization potential, is the energy necessary to. What is lowest ionization energy? Thus, helium has the largest first ionization energy, while francium has one of the lowest.
 Source: quora.com
Source: quora.com
Which has lowest ionization energy? Which best explains why ionization energy tends to decrease from the top to the bottom of a group? Ionisation energy increases as we move left to right across a period (i.e., from l i to n e) and decreases when from top to bottom in a group.
 Source: clutchprep.com
Source: clutchprep.com
Thus, helium has the largest first ionization energy, while francium has one of the lowest. Hence sodium (electronic configuration =1s22s22p63s1) has the lowest ionisation energy. Compared to the ionization energy of a magnesium atom, the ionization energy of a calcium atom is smaller, this is primarily because the calcium atom:

Ionization energy value decreases from top to bottom in a group because the shielding effect in atoms increases as we move down a group. Ionization energy is the minimum amount of energy required to remove the electron from outermost shell (termed as first ionization energy) of an isolated atom. Compared to the ionization energy of a magnesium atom, the ionization energy of a calcium atom is smaller, this is primarily because the calcium atom:
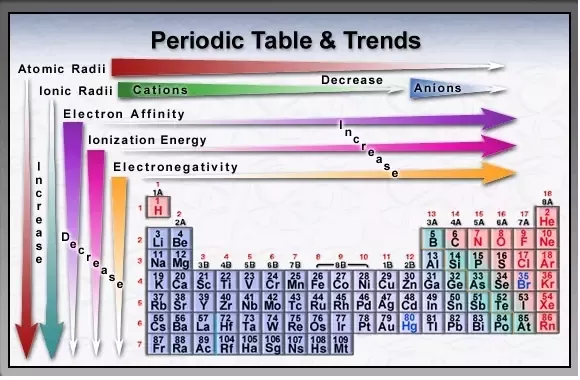 Source: quora.com
Source: quora.com
Thus, helium has the largest first ionization energy, while francium has one of the lowest. Alkali metalsthe group of elements which have the lowest ionization energy are the alkali metals. Which has low ionization potential?

Cesiumfrom this trend, cesium is said to have the lowest ionization energy and fluorine is said to have the highest ionization energy (with the exception of helium and neon). From lowest energy to highest energy which of the following correctly orders the different list the following atoms in order of increasing ionization energy: Thus, helium has the largest first ionization energy, while francium has one of the lowest.
Also Read :