The decrease of entropy in the penny is less than the increase in entropy of the water. 2 so2 (g)+ o2 (g) → 2 so3 (g) 248.1 205.0 256.6.
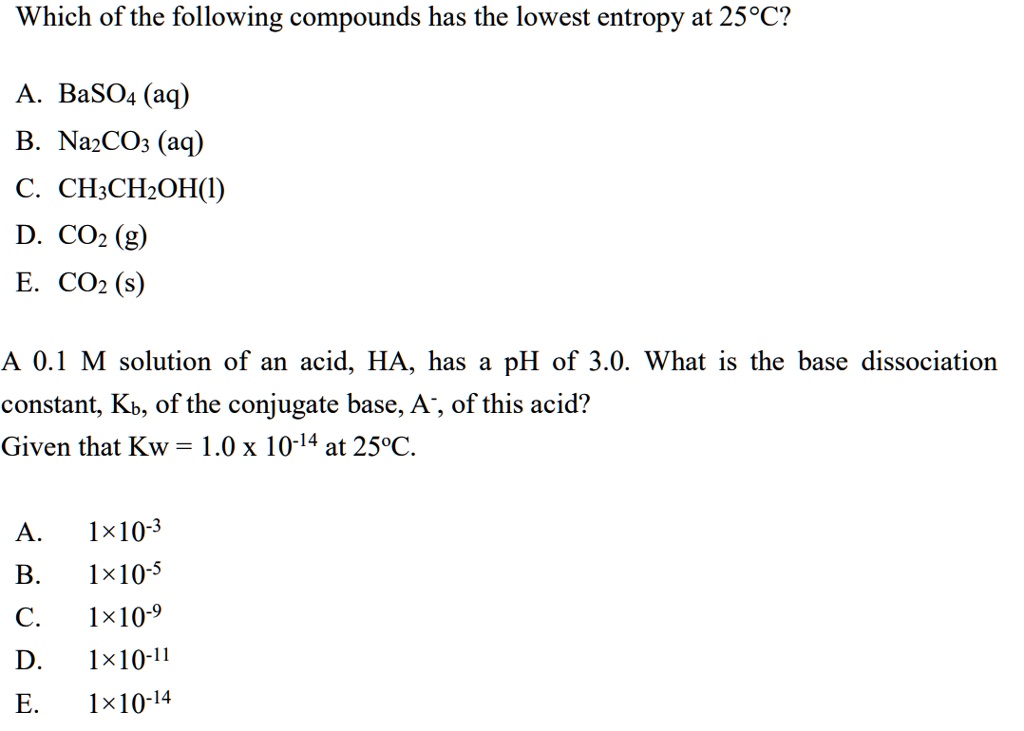
Which Of The Following Has The Lowest Entropy. G is equal to h at stp, therefore: Which of the following states of matter should have the lowest entropy value? Thus, we can conclude that out of the given options, has the lowest entropy. When a substance is a gas it has many more microstates and thus have the highest entropy.
 Solved Question 4 Which Of The Following Has The Lowest | Chegg.com From chegg.com
Solved Question 4 Which Of The Following Has The Lowest | Chegg.com From chegg.com
Related Post Solved Question 4 Which Of The Following Has The Lowest | Chegg.com :
Liquids have more microstates (since the molecules can translate) and thus have a higher entropy. The decrease of entropy in the penny is less than the increase in entropy of the water. Thus, they are not moving and hence in solids entropy is the lowest. Indicate which of the following has the lowest standard molar entropy (s°).
All have the same entropy.
Asked jan 21 in biology & microbiology by kplecker G is equal to h at stp, therefore: Solution for which of the following has the lowest entropy (all at 20.0℃)? Which of the following has a negative entropy? 2 mol of hcn e. When a substance is a gas it has many more microstates and thus have the highest entropy.
![Solved] Which Of The Following Has The Lowest Entropy?](https://storage.googleapis.com/tb-img/production/21/03/F1_Puja.J__26-02-21_Savita_D3.png “Solved] Which Of The Following Has The Lowest Entropy?") Source: testbook.com
Asked sep 17, 2016 in chemistry by messi10. All have the same entropy. Liquids have more microstates (since the molecules can translate) and thus have a higher entropy.
 Source: clutchprep.com
Source: clutchprep.com
1 mol of hcn c. 1 mol of hcn c. 0.5 g of hcn b.
![Solved] Which Of The Following Has The Lowest Entropy?](https://storage.googleapis.com/tb-img/production/21/03/F1_Puja.J__26-02-21_Savita_D4.png “Solved] Which Of The Following Has The Lowest Entropy?") Source: testbook.com
A) ch4 (g) b) c2h2 (g) c) c2h4 (g) d) c2h6 (g) e). When a substance is a gas it has many more microstates and thus have the highest entropy. Ch30h (g, 15°c) which of the following gas molecules has the highest standard molar entropy at 25°c?
 Source: numerade.com
Source: numerade.com
A) ch4 (g) b) c2h2 (g) c) c2h4 (g) d) c2h6 (g) e). Which one of the following would be expected to have the lowest standard molar entropy, s°, at 25°c? A) ch4 (g) b) c2h2 (g) c) c2h4 (g) d) c2h6 (g) e).
 Source: chegg.com
Source: chegg.com
Asked sep 17, 2016 in chemistry by messi10. When a substance is a gas it has many more microstates and thus have the highest entropy. An isolated system spontaneously moves toward dynamic equilibrium (maximum entropy) so it constantly is transferring energy between components and increasing its entropy.

Entropy is highly involved in the second law of thermodynamics: Which of the following has the lowest entropy? Entropy is the quantitative measure of spontaneous processes and how energy disperses unless actively stopped from doing so.
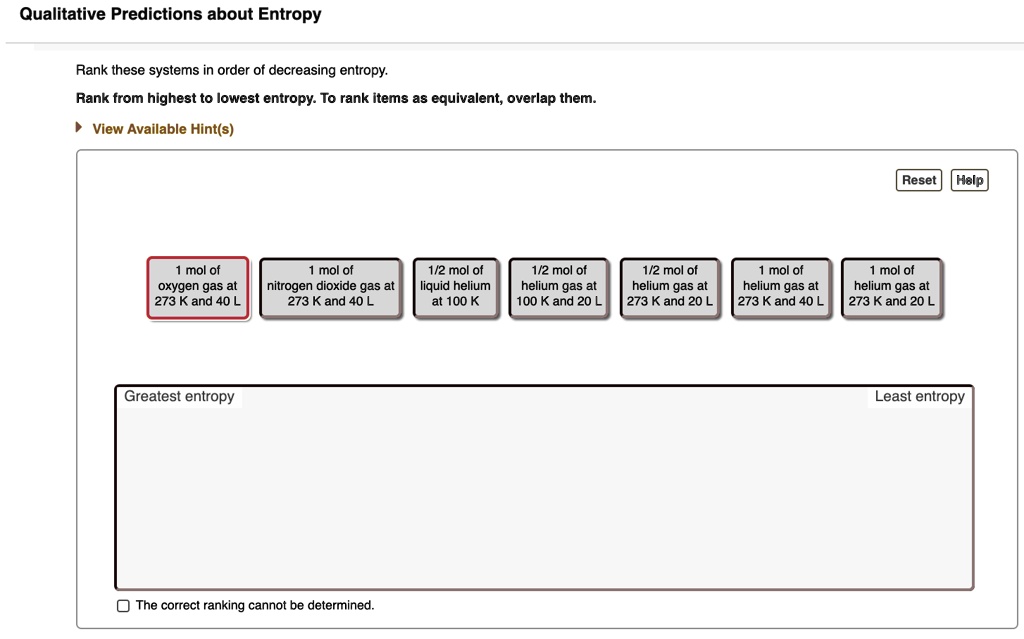 Source: itprospt.com
Source: itprospt.com
Standard molar entropies, s°, in j/kmol, are given below each reactant and product in the reaction shown below. Spontaneous only at high temepratures. 1 mol of hcn c.
 Source: chegg.com
Source: chegg.com
Which one of the following has the lowest standard molar entropy; The standard entropy of reaction, δs°, for this reaction is ________ j/k. Iz(g) nz(g) fzlg) clzlg) iz(s) submit request answer get the answer to your homework problem.
 Source: chegg.com
Source: chegg.com
The decrease of entropy in the penny is less than the increase in entropy of the water. G as > l iq u id. Thus, they are not moving and hence in solids entropy is the lowest.
 Source: chegg.com
Source: chegg.com
All of the above have the same entropy at 298 k. 3 show answers another question on chemistry. An isolated system spontaneously moves toward dynamic equilibrium (maximum entropy) so it constantly is transferring energy between components and increasing its entropy.
 Source: clutchprep.com
Source: clutchprep.com
When a substance is a gas it has many more microstates and thus have the highest entropy. Which one of the following would be expected to have the lowest standard molar entropy, s°, at 25°c? The decrease of entropy in the penny is less than the increase in entropy of the water.
 Source: chegg.com
Source: chegg.com
B.) none of the above. When a substance is a gas it has many more microstates and thus have the highest entropy. C.) raising the temperature of 1l of water to 2950c to 3000 c.

Compare the genetic makeup of liver cells and pancreatic cells. 2 kg of hcn d. Hcl (s), hcl (g), hcl (l), hbr (g), hi (g) the entropy of a substance in.

Homework answers / question archive / chapter 17: Arrange the following substances in the order of increasing entropy at 25° c. Thus, we can conclude that out of the given options, has the lowest entropy.
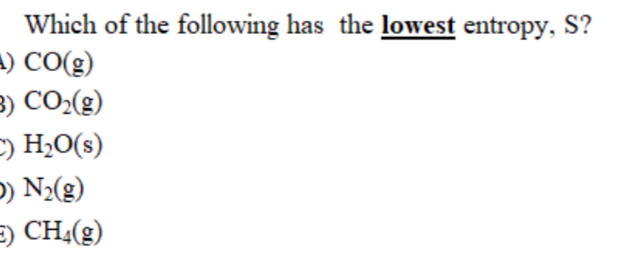 Source: chegg.com
Source: chegg.com
2 mol of hcn e. The decrease of entropy in the penny is less than the increase in entropy of the water. Which of the following has the lowest entropy?

A.) evaporationg one mole of a liquid. 3 show answers another question on chemistry. Thus, they are not moving and hence in solids entropy is the lowest.
 Source: bartleby.com
Source: bartleby.com
Entropy, free energy, and equilibrium 1)which of these species would you expect to have the lowest standard entropy (s°)? H is +137 kj/mol and s is +120 j/k mol. Thus, they are not moving and hence in solids entropy is the lowest.
 Source: numerade.com
Source: numerade.com
Indicate which of the following has the lowest standard molar entropy (s°) a. Molar entropy is the amount of randomness in one mole of a substance. A) ch4 (g) b) c2h2 (g) c) c2h4 (g) d) c2h6 (g) e).
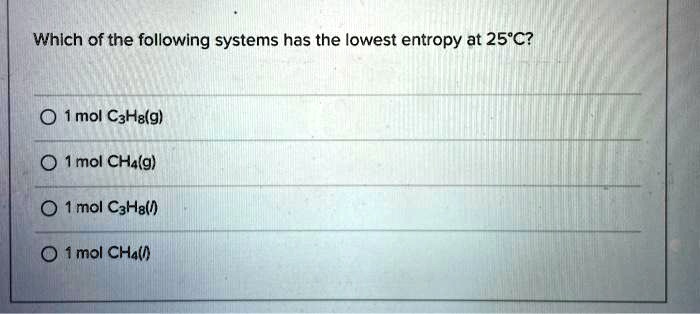 Source: numerade.com
Source: numerade.com
G as > l iq u id. 1 mol of hcn c. Solids have the fewest microstates and thus the lowest entropy.
 Source: slideplayer.com
Source: slideplayer.com
C10h22(s) which one of the following has the lowest standard molar entropy, s°, at 25°c? Entropy is highly involved in the second law of thermodynamics: When a substance is a gas it has many more microstates and thus have the highest entropy.
Also Read :





