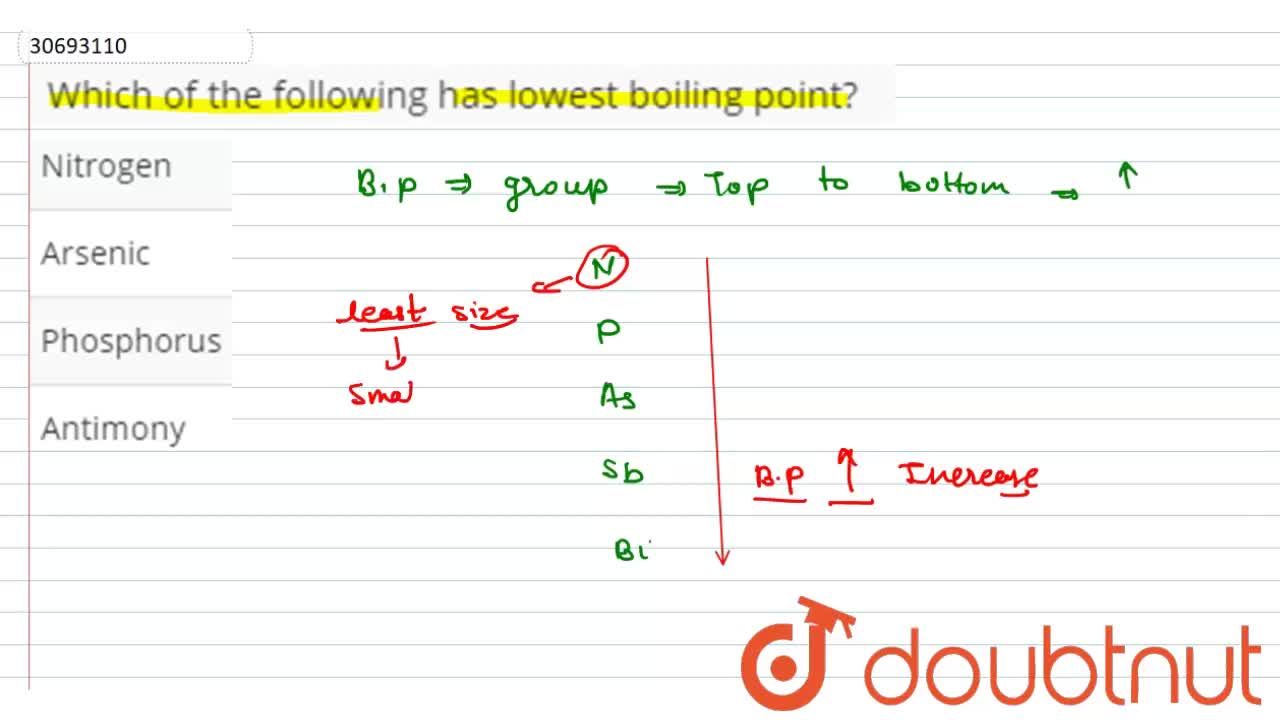
Among the following select the alkane that is expected to have lowest boiling point. A) h20 b) n2 c) soz d) h2 e) co2 which one of the following correctly ranks the compounds in order of lowest boiling point to highest boiling point based only in.
Which Of The Following Has The Lowest Boiling Point. The unity used for the melting point is celsius (c). The chemical element with the lowest boiling point is helium and the element with the highest boiling point is tungsten. I will give you a general way that will help you to know not only the below answer but any answer the below picture is the structure of pentane they are all aligned in the parent chain which is a very strong bond and donot dissociate properly another picture is the picture of. The magnitude of london dispersion forces decreases with a decrease in molecule size (carbon chain length and molecular surface area).
 Solved Which Of The Following Has The Lowest Boiling Point? | Chegg.com From chegg.com
Solved Which Of The Following Has The Lowest Boiling Point? | Chegg.com From chegg.com
Related Post Solved Which Of The Following Has The Lowest Boiling Point? | Chegg.com :
What does negative melting point mean? 5)of the following substances, _____ has the highest boiling point. Which one of the following alkyl halides has the lowest boiling point. It has the highest boiling points next comes methanol, ch_4o or ch_3oh.
What does negative melting point mean?
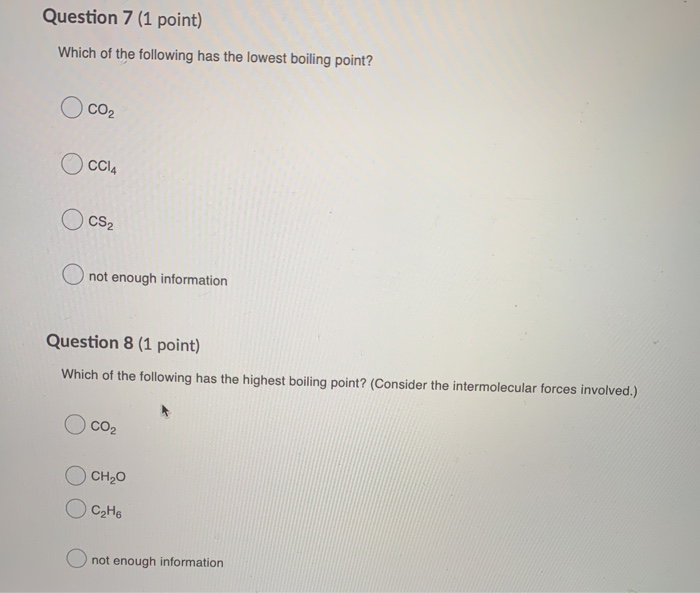
5)of the following substances, _____ has the highest boiling point. N2 br2 h2 cl2 o2 a)o2 b)br2 c)n2 d)h2 e)cl2 6) 7)in which of the following molecules is hydrogen bonding likely to be the most significant The magnitude of london dispersion forces decreases with a decrease in molecule size (carbon chain length and molecular surface area). The unity used for the melting point is celsius (c). Previous losses follow a uniform distribution from 0 to 20,000. Halogen literally means �salt producing� since thes.
 Source: doubtnut.com
Source: doubtnut.com
5)of the following substances, _____ has the highest boiling point. Let�s go to the basics; Part b and c are done the same way.
 Source: chegg.com
Source: chegg.com
What does negative melting point mean? Due to which the attractive forces between the molecules (van der waals forces) decreases. The chemical element with the lowest boiling point is helium and the element with the highest boiling point is tungsten.
 Source: youtube.com
Source: youtube.com
If the boiling point is below 20 o c, then the liquid has already boiled and is a gas. Heliumthe chemical element with the lowest boiling point is helium and the element with the highest boiling point is tungsten. Therefore, the shortest, most branched molecule in this problem will have the lowest boiling point.
 Source: quizzclub.com
Source: quizzclub.com
For isomeric alcohols, the boiling point decreases with increase in branching of carbon chain. N2 br2 h2 cl2 o2 a)o2 b)br2 c)n2 d)h2 e)cl2 6) 7)in which of the following molecules is hydrogen bonding likely to be the most significant Which one of the following correctly ranks the compounds in order of lowest enthalpy of vaporization to highest enthalpy of vaporization based only in intermolecular forces?
 Source: toppr.com
Source: toppr.com
Hf only has 8 electrons, but its ability to form hydrogen bonds results in a higher boiling point. List the following molecules in order of increasing boiling point: Hf only has 8 electrons, but its ability to form hydrogen bonds results in a higher boiling point.
 Source: oneclass.com
Source: oneclass.com
Which compound has the lowest boiling point? If the boiling point is below 20 o c, then the liquid has already boiled and is a gas. The correct answer is isobutane, a four membered, branched hydrocarbon.
 Source: seniorcare2share.com
Source: seniorcare2share.com
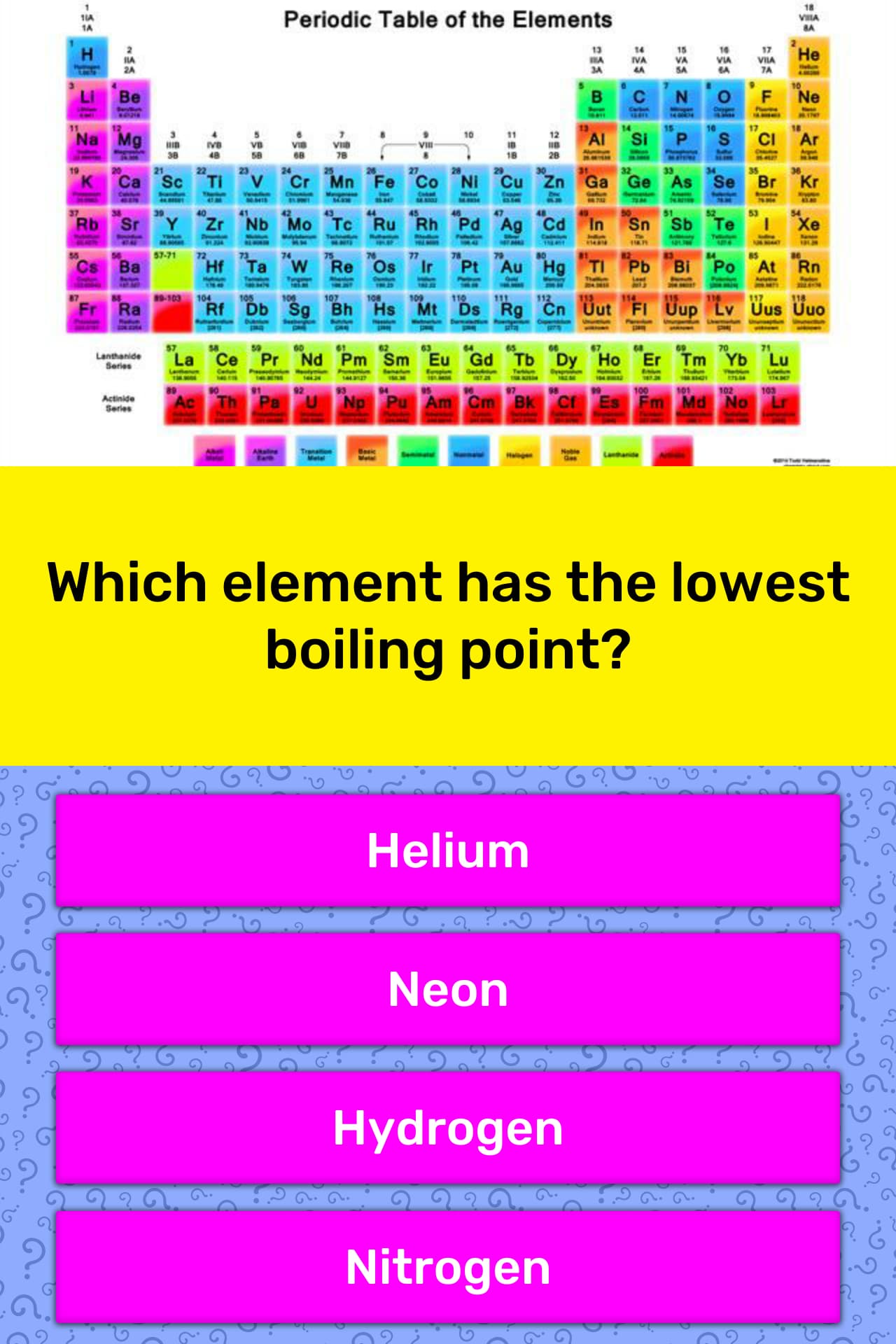
It has been found that helium has the lowest normal boiling point (−268.9 °c) because it has very weak intermolecular attractions. Which has lowest boiling point? So the highest boiling point will be the one that has the highest i*m which is na2so4.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
The unity used for the melting point is celsius (c). I for ethylene glycol is 1. A) h20 b) n2 c) soz d) h2 e) co2 which one of the following correctly ranks the compounds in order of lowest boiling point to highest boiling point based only in.
 Source: chegg.com
Source: chegg.com
Which of the following hydrides has the lowest boiling point h2o h2s h2se. Which compound has the lowest boiling point? Due to which the attractive forces between the molecules (van der waals forces) decreases.
 Source: studylib.net
Source: studylib.net
.fo 9) which of the following alkynes has the lowest boiling point? The halogens in the periodic table. If their policy has a deductible of $1,000, what is their.
 Source: youtube.com
Source: youtube.com
Due to which the attractive forces between the molecules (van der waals forces) decreases. At room temperature, the lighter alkanes are gases; I for bacl2 is 3.
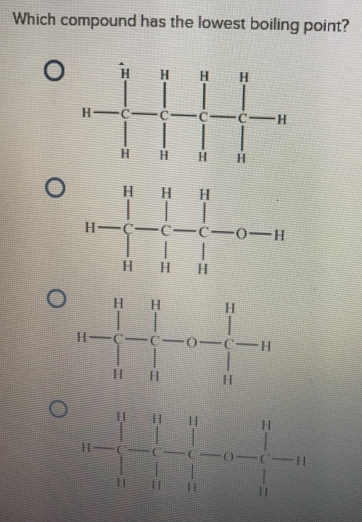 Source: clutchprep.com
Source: clutchprep.com
The magnitude of london dispersion forces decreases with a decrease in molecule size (carbon chain length and molecular surface area). Therefore diphenyl ether which has a molecular weight of 170 g/mol has much stronger london dispersion forces as compared to diethyl ether which has a molecular weight of 74 g/mol from above discussion, we can conclude that diethyl ether has the weakest intermolecular forces of attraction. Nh3 > sbh3 > ash3 > ph3.
![Solved] Which Compound Has The Lowest Boiling Point? | Course Hero](https://www.coursehero.com/qa/attachment/16046178/ “Solved] Which Compound Has The Lowest Boiling Point? | Course Hero”) Source: coursehero.com
Heliumthe chemical element with the lowest boiling point is helium and the element with the highest boiling point is tungsten. Please log in or register to add a comment. Therefore, the shortest, most branched molecule in this problem will have the lowest boiling point.
 Source: doubtnut.com
Source: doubtnut.com
The tabular chart on the right is arranged by. Please log in or register to add a comment. The correct answer is isobutane, a four membered, branched hydrocarbon.
 Source: study.com
Source: study.com
Go through the list above. .fo 9) which of the following alkynes has the lowest boiling point? Hence it has the lowest boiling point.
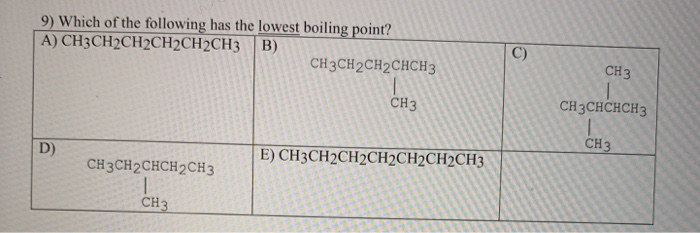 Source: chegg.com
Source: chegg.com
The midweight alkanes are liquids; N2 br2 h2 cl2 o2 a)o2 b)br2 c)n2 d)h2 e)cl2 6) 7)in which of the following molecules is hydrogen bonding likely to be the most significant Nh3 will have the highest boiling point because nh3 molecules can form hydrogen bonds with each other.
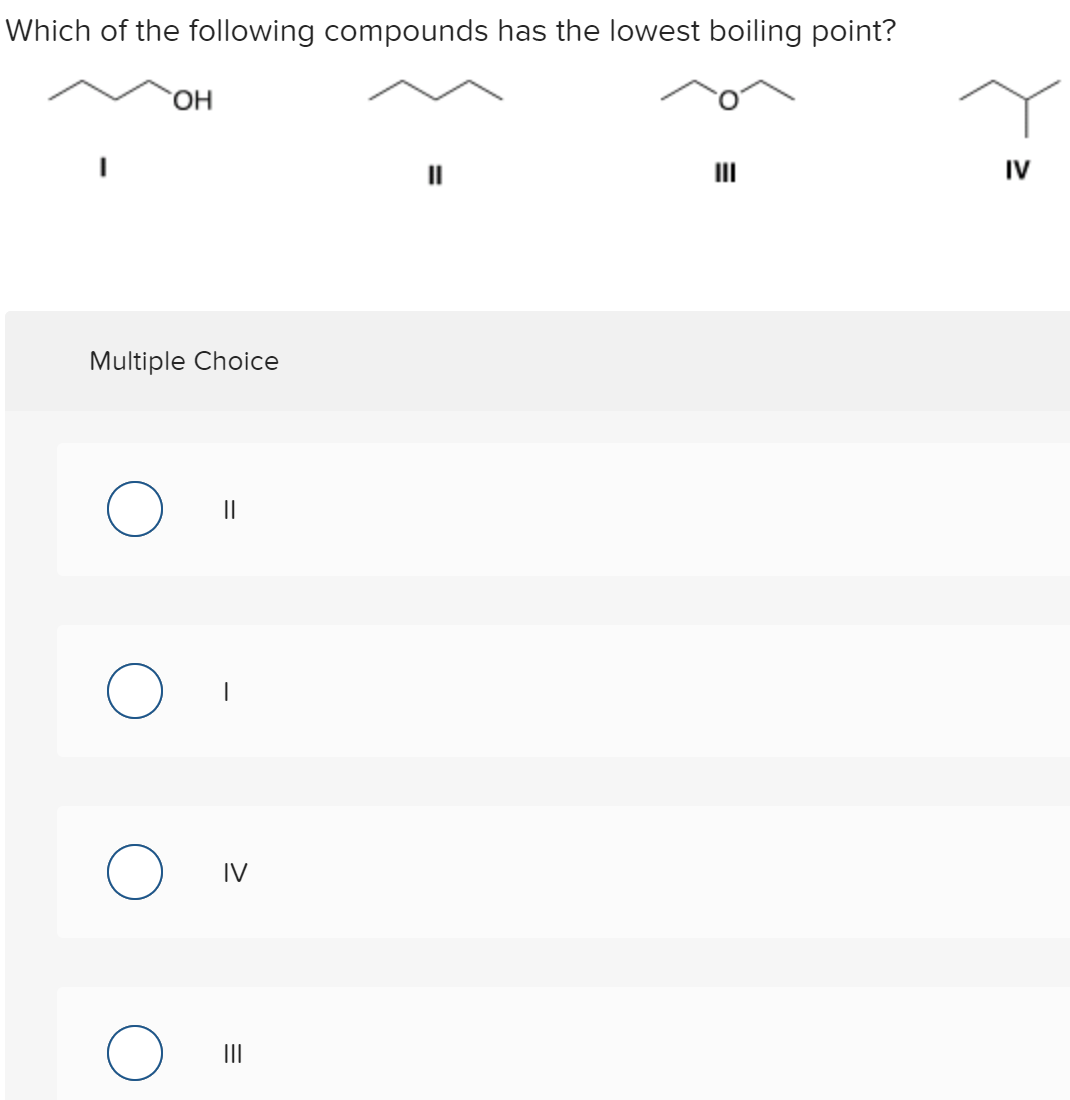 Source: bartleby.com
Source: bartleby.com
I will give you a general way that will help you to know not only the below answer but any answer the below picture is the structure of pentane they are all aligned in the parent chain which is a very strong bond and donot dissociate properly another picture is the picture of. Hence it has the lowest boiling point. What is the the boiling point trend in terms of the molecular weights of the compounds?

Which of the following has the lowest boiling point? List the following molecules in order of increasing boiling point: Among the following select the alkane that is expected to have lowest boiling point.
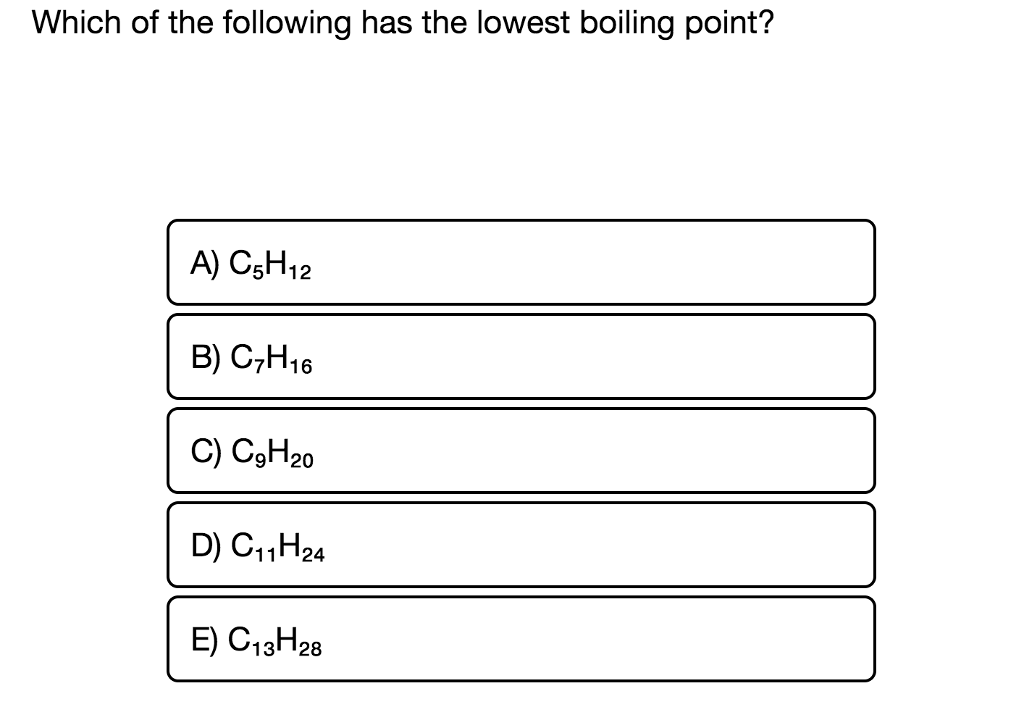 Source: chegg.com
Source: chegg.com
Br2, f2, i2, cl2, answer higher boiling points will correspond to stronger intermolecular forces. Among the following select the alkane that is expected to have lowest boiling point. The magnitude of london dispersion forces decreases with a decrease in molecule size (carbon chain length and molecular surface area).
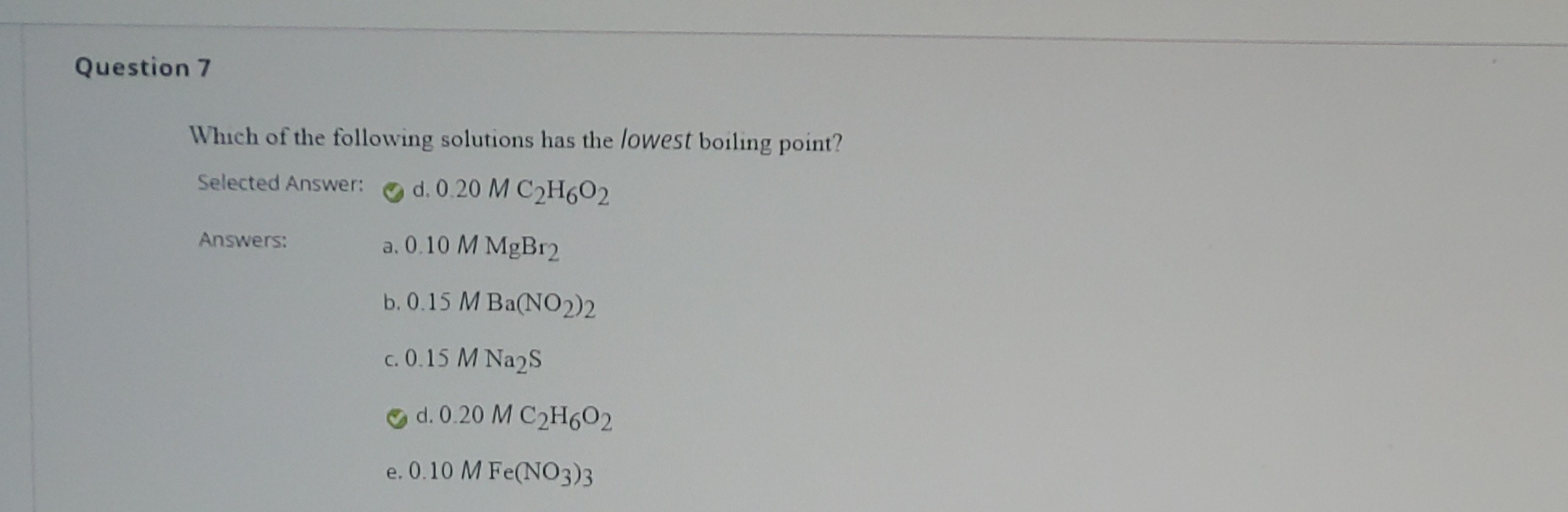 Source: clutchprep.com
Source: clutchprep.com
None of these have hydrogen bonding. The unity used for the melting point is celsius (c). The unity used for the melting point is celsius (c).
Also Read :