Which of the following atoms has the smallest radius? Does al or al3+ have a larger radius?
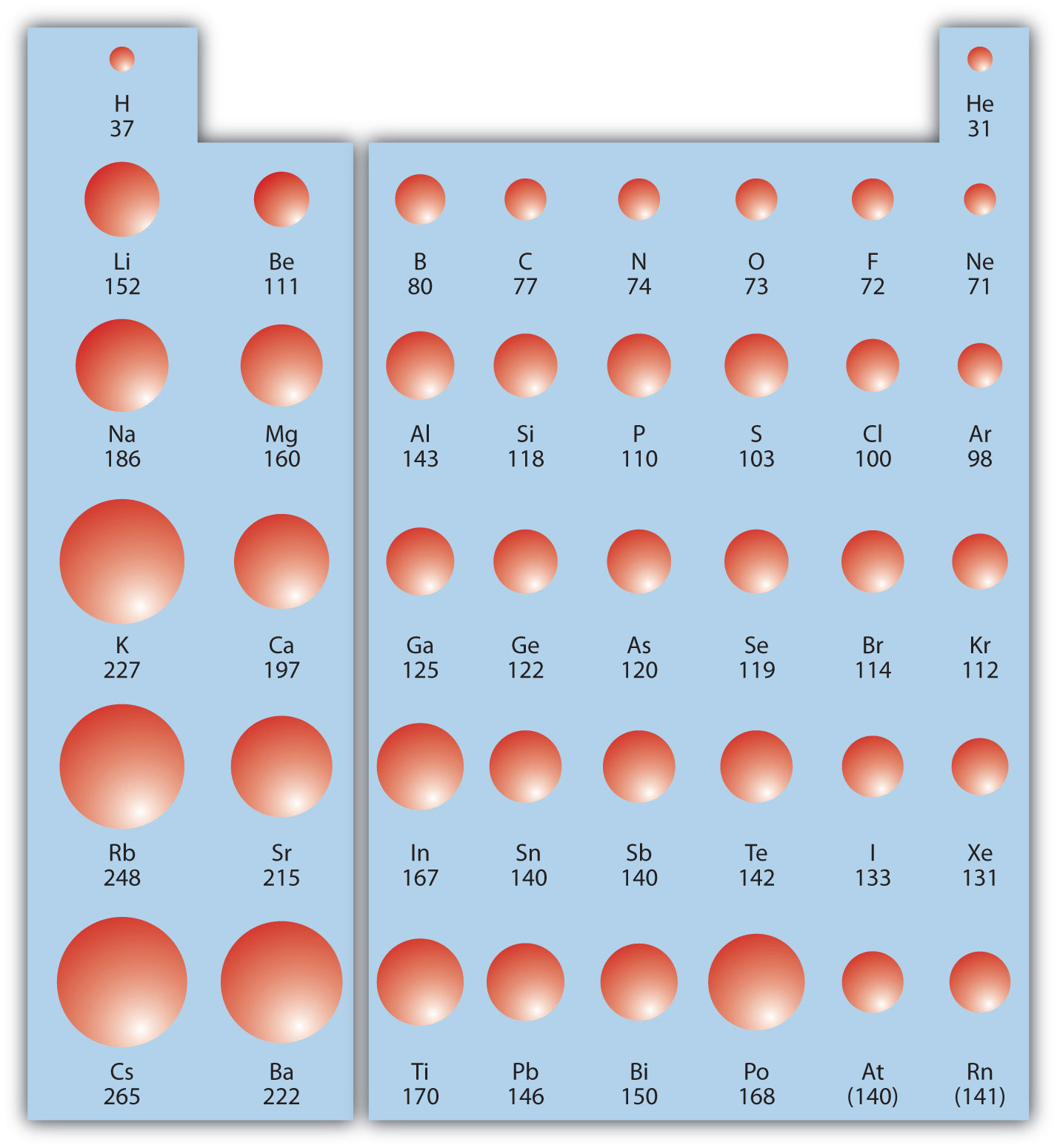
Which Of The Following Has The Largest Radius. Magnesium has one more proton than sodium, and therefore, with the same number of electrons and 1s orbital shielding, the zeff for magnesium is greater, and therefore mg2+ has the smaller ionic radius. 6) which one of the following atoms has the largest radius? Li > c > o > f. Therefore, 1 s 2, 2 s 2, 2 p 6, 3 s 2 has the largest radius.
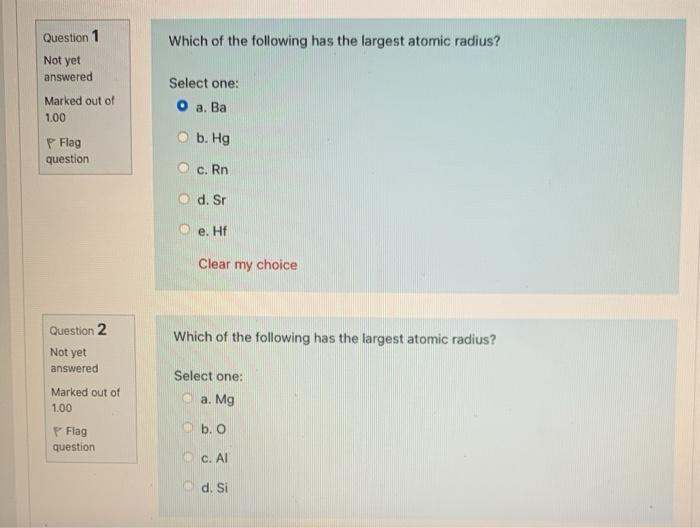
Related Post Solved Question 1 Which Of The Following Has The Largest | Chegg.com :
This answer is not useful. So, the correct answer is option c. As the nuclear charge increases the attractive force between nucleus and electrons increases. This will compress the energy levels a bit and make the ionic radius smaller for the potassium cation.
How do ions compare in size?
So we have f minus or two minus b plus three. Thus, the order of increasing radius is n lt c lt p lt si. Which of the following has the largest radius? So selenium as the greatest radius because it belongs to the fourth period while the others belonged to the third. The periodic trend goes, from smallest to largest, from the top right to the bottom left. This will compress the energy levels a bit and make the ionic radius smaller for the potassium cation.
 Source: toppr.com
Source: toppr.com
A) rb b) si c) s d) o. Which of the following atoms has the largest radius? A) i b) co c) ba d) sr e) ca
 Source: toppr.com
Source: toppr.com
How do ions compare in size? A) na b) ba c) ca d) cs. These ions each have achieved the nearest noble gas configuration.
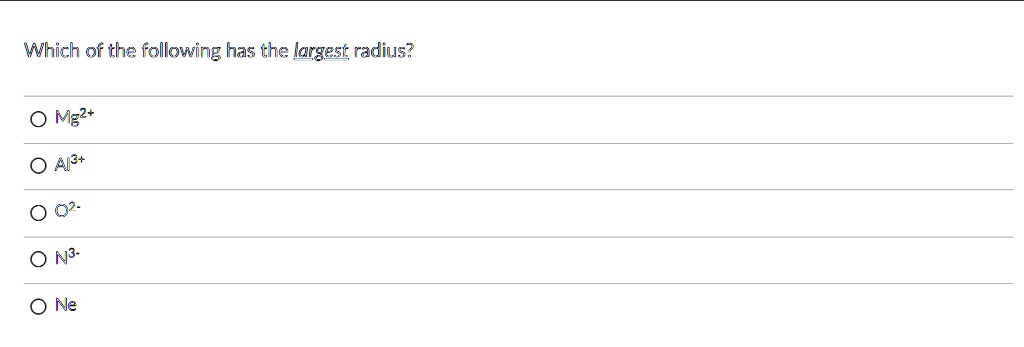 Source: numerade.com
Source: numerade.com
As a result orbital electrons become more closer to nucleus. Which of the following species has the largest radius m | which of the following species has the largest radius? Therefore, rubidium has the largest atomic radius whereas helium has the smallest.
 Source: toppr.com
Source: toppr.com
A) sr b) ca c) y d) rb e) k They increase from top to bottom and from right to left in the periodic table. Which of the following species has the largest radius m | which of the following species has the largest radius?
 Source: youtube.com
Source: youtube.com
Sulfur and also chlorine are in the shortest period, for this reason they have actually the biggest atomic radii. Cations are smaller than their parent atoms because of increase in nuclear charge. Therefore, the chloride anion will have the larger atomic radius.

Does mg2+ have a larger radius than na+? Which of the following atoms has the smallest radius? These ions each have achieved the nearest noble gas configuration.
 Source: brainly.com
Source: brainly.com
Which atom has the highest first ionization energy? Question is which one of the following iron has done? Li > c > o > f.
 Source: toppr.com
Source: toppr.com
Therefore, the radius of n is smaller than that of c (atomic radius decreases as we move from left to right across a period). Which of the following has the largest atomic radius? I used the value of an ingredients.
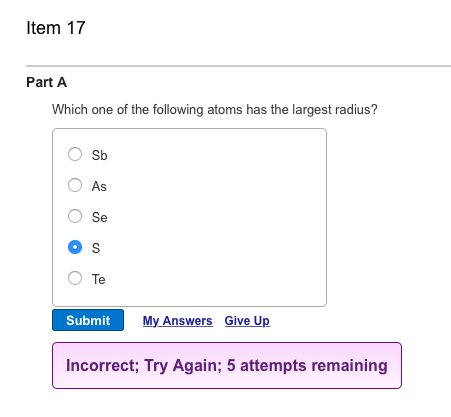 Source: chegg.com
Source: chegg.com
The first electron will circle that at some distance, making a h atom. Atomic radius of hydrogen (h) 120. A) rb b) si c) s d) o.

119 rows atomic radius of elements (pm) 1: A) cs b) ca c) al d) o. I used the value of an ingredients.
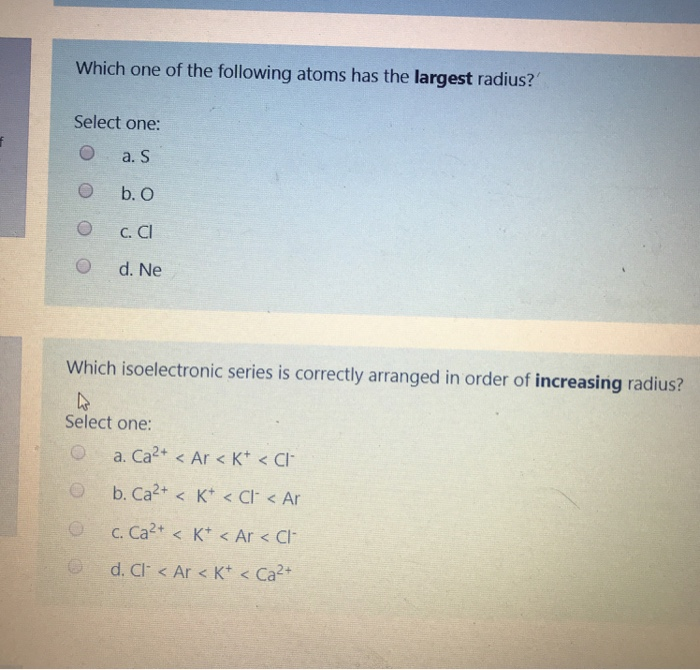 Source: chegg.com
Source: chegg.com
Li > c > o > f. A) cs b) ca c) al d) o. Which atom has the lowest first ionization energy?
 Source: chegg.com
Source: chegg.com
The ionic radii of cations follow the same trends as atomic radii. In other words, k+ has bigger effective nuclear charge than cl−, which translates to a bigger net positive charge felt by the outermost electrons. (©) 1s?, 2s?, 2p�, 3s, 3p3 (d) 1s2, 2s2, 2p, 3s2, 3ps arrange the elements in increasing order of atomic radius
 Source: oneclass.com
Source: oneclass.com
A) na b) al c) n d) f. 119 rows atomic radius of elements (pm) 1: Show activity on this post.
 Source: socratic.org
Source: socratic.org
Thus atomic radius is directly proportional to the number of electron shells. Which of the following species has the largest radius m | which of the following species has the largest radius? Question is which one of the following iron has done?

This homework question can be solved by thinking about how the charges react to each other. Which atom has the lowest first ionization energy? Which atom has the largest negative electron affinity?
 Source: clutchprep.com
Source: clutchprep.com
Well, i only see two elements out of the four options, but out of those two, cl has a larger atomic radius. A) sr b) ca c) y d) rb e) k These ions each have achieved the nearest noble gas configuration.
 Source: youtube.com
Source: youtube.com
Hence, li has the largest atomic radius and f has the smallest atomic radius. Which atom has the lowest first ionization energy? Thus ions will have radii different from the atoms because ions will have either gained or lost electrons.

Which atom has the highest first ionization energy? Then the second part we are minus has the largest radius. Because of the charge present on it.
 Source: chegg.com
Source: chegg.com
So, the correct answer is option c. A) o b) f c) s d) cl e) ne is isoelectronic with argon and is isoelectronic with neon. Of the atoms here, the br atom would be the largest as it is farthest down the group and hence its anion also will be the largest ion.
So, the correct answer is option c. The ionic radii of cations follow the same trends as atomic radii. Well, i only see two elements out of the four options, but out of those two, cl has a larger atomic radius.
Also Read :





