1−butyne has larger dipole moment because the electronegativity of sp−c is more than that of sp 2−c. H2 h2o h2s h2se ch4
Which Of The Following Has The Largest Dipole Moment. $\mu = ,{\text{q}}{\text{.d}}$ where, $\mu ,$ is the dipole moment. These molecules have tetrahedral geometry due to s p 3 hybridization of carbon atom. Hf has the largest dipole moment, you can tell which molecule has the largest by looking on the periodic table, they are usually the pair that are furthest from each other and it is also due to them having the biggest difference in electronegativity, usually the closer two elements are, the weaker the dipole moment. C h 3f c h 3 f.
 Solved Which Of The Following Compounds Would You Expect To | Chegg.com From chegg.com
Solved Which Of The Following Compounds Would You Expect To | Chegg.com From chegg.com
Related Post Solved Which Of The Following Compounds Would You Expect To | Chegg.com :
C h 3f c h 3 f. Μ c c l 4 = 0, μ c h c l 3 = 1. Dichloromethane has highest dipole moment among c h 2 c l 2 , c h c l 3 and c c l 4. Since br is above i, this means that br is more electronegative, thus making hbr more electronegative than hi.
7 posts • page 1 of 1.
0 d, μ c h 2 c l 2 = 1. Why is the dipole moment of ccl4 zero? Check answer and solution for above question from chemistry in t Hence, b is the right answer. Asked apr 7, 2018 in chemistry by shabnam praween (138k points) which of the following species has the highest dipole moment? Dipole moment is given by charge * distance.
 Source: toppr.com
Source: toppr.com
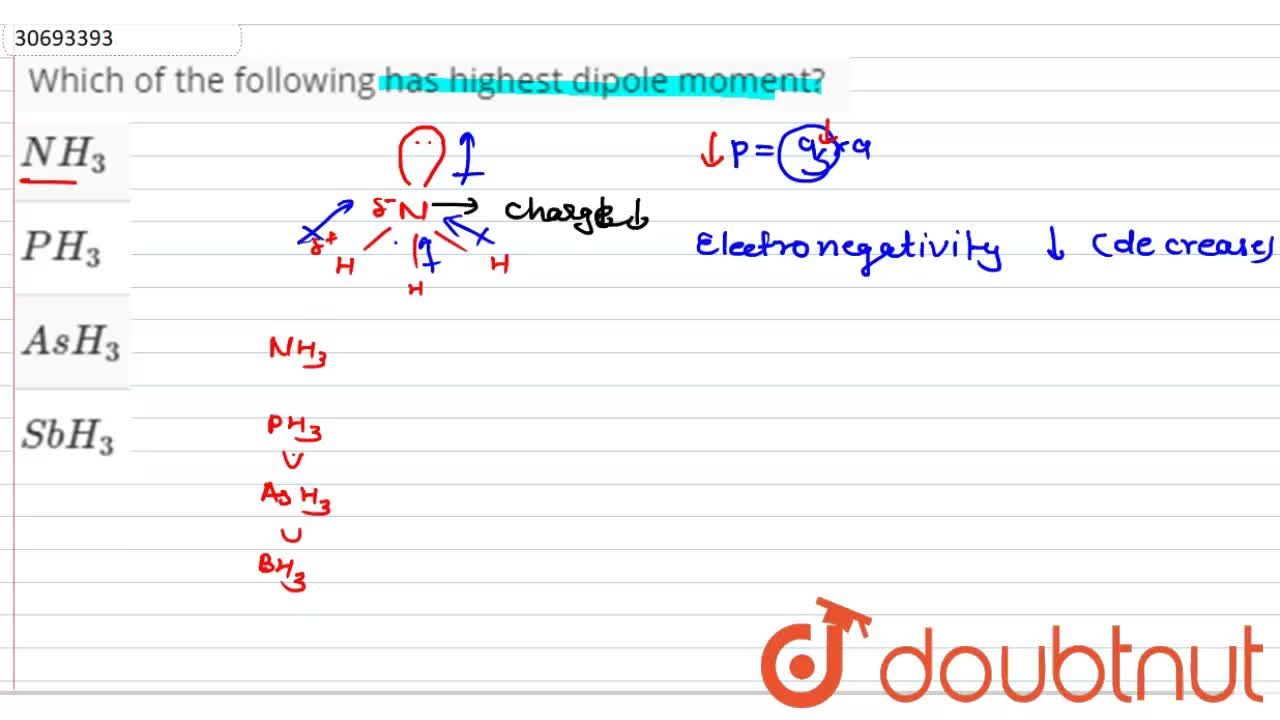
These molecules have tetrahedral geometry due to s p 3 hybridization of carbon atom. (a) nh3 (b) ph3 (c) ash3 (d) sbh3. Compounds with stronger intermolecular forces will have higher boiling points (ion ion > hydrogen bonding > dipole dipole > london dispersion).
 Source: slideplayer.com
Source: slideplayer.com
$\mu = ,{\text{q}}{\text{.d}}$ where, $\mu ,$ is the dipole moment. 7 posts • page 1 of 1. Click to see full answer.
 Source: chegg.com
Source: chegg.com
C h 3f c h 3 f. For this reason no polarity. Note:dipole moment can occur between two ions in an ionic bond or between atoms in a covalent bond.
 Source: youtube.com
Source: youtube.com
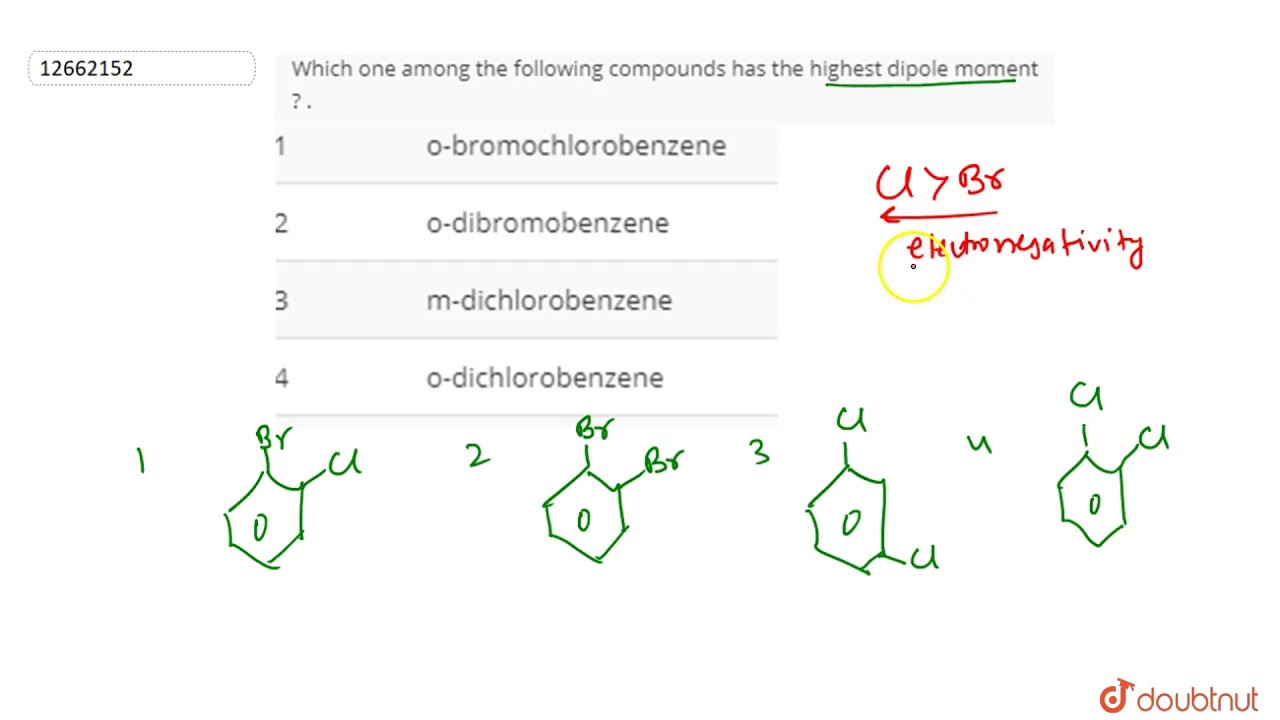
Electronegativity in halogens decreases from top to bottom. Br2 is nonpolar and only has dispersion forces. Dipole moment in the molecule depends upon the charge and the distance between the charges.
 Source: eanshub.com
Source: eanshub.com
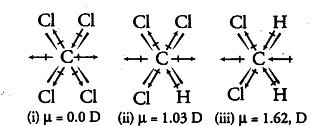
The formula to calculate the magnitude of dipole moment is as follows: Chcl3 has highest dipole moment because eventhough its charge is less than that of flourine its distance is cosiderably greater. Thus, c h 2 c l 2 has a dipole moment = 1.62 d.
 Source: clutchprep.com
Source: clutchprep.com
Br2 is nonpolar and only has dispersion forces. Hence, the given compounds can be arranged in the increasing order of their dipole moments as ccl 4 < chcl 3 < ch 2 cl 2 Hf has the largest dipole moment, you can tell which molecule has the largest by looking on the periodic table, they are usually the pair that are furthest from each other and it is also due to them having the biggest difference in electronegativity, usually the closer two elements are, the weaker the dipole moment.
 Source: goiit.com
Source: goiit.com
Hence, $c{{(h)}{2}}{{(cl)}{2}}$ has the highest dipole moment amongst the given options. The atoms are in a tetrahedron with the carbon in the centre. Which of the following covalent bonds has the largest.
 Source: ask.learncbse.in
Source: ask.learncbse.in
The dipole moment of both nitro and amine group are in the same direction so they add up which increases the dipole moment. 0 d, μ c h 2 c l 2 = 1. (a) nh 3 (b) ph 3 (c) ash 3 (d) sbh 3.

Please log in or register to add a comment. $\mu = ,{\text{q}}{\text{.d}}$ where, $\mu ,$ is the dipole moment. Based on this information, choose the response that best completes the following sentence:
 Source: meritnation.com
Source: meritnation.com
As the dipole moment has both magnitude and direction, so it is a vector quantity. Hence, $c{{(h)}{2}}{{(cl)}{2}}$ has the highest dipole moment amongst the given options. Μ = e × d, where μ is the bond dipole moment, e is the amount of charge which is separated, and d is the distance over which the charge is separated.
 Source: doubtnut.com
Source: doubtnut.com
Hence, b is the right answer. Please log inor registerto add a comment. As oxygen is more electronegative than nitrogen, iodine or sulphur, h2o will have greater dipole moment.
 Source: chegg.com
Source: chegg.com
The dipole moment of both nitro and amine group are in the same direction so they add up which increases the dipole moment. The decreasing order of dipole moments is c h 2 c l 2 > c h c l 3 > c c l 4. Asked apr 7, 2018 in chemistry by shabnam praween (138k points) which of the following species has the highest dipole moment?
 Source: youtube.com
Source: youtube.com
Note:dipole moment can occur between two ions in an ionic bond or between atoms in a covalent bond. Which of the following has the largest dipole moment? Ccl 4 < chcl 3 < ch 2 cl 2.
 Source: doubtnut.com
Source: doubtnut.com
Dichloromethane has highest dipole moment among c h 2 c l 2 , c h c l 3 and c c l 4. These molecules have tetrahedral geometry due to s p 3 hybridization of carbon atom. The decreasing order of dipole moments is c h 2 c l 2 > c h c l 3 > c c l 4.
 Source: toppr.com
Source: toppr.com
As the dipole moment has both magnitude and direction, so it is a vector quantity. As oxygen is more electronegative than nitrogen, iodine or sulphur, h2o will have greater dipole moment. As a result, ch 2 cl 2 has a higher dipole moment of 1.60 d than chcl 3 i.e., ch 2 cl 2 has the highest dipole moment.
 Source: chegg.com
Source: chegg.com
Please log in or register to add a comment. Electronegativity in halogens decreases from top to bottom. C h 3f c h 3 f.
 Source: chegg.com
Source: chegg.com
(a) nh 3 (b) ph 3 (c) ash 3 (d) sbh 3. Br2 is nonpolar and only has dispersion forces. $\mu = ,{\text{q}}{\text{.d}}$ where, $\mu ,$ is the dipole moment.
 Source: youtube.com
Source: youtube.com
These molecules have tetrahedral geometry due to s p 3 hybridization of carbon atom. For this reason no polarity. Please log in or register to add a comment.
 Source: numerade.com
Source: numerade.com
1−butyne has larger dipole moment because the electronegativity of sp−c is more than that of sp 2−c. The decreasing order of dipole moments is c h 2 c l 2 > c h c l 3 > c c l 4. Which of the following covalent bonds has the largest.

Ch3f has the highest dipole and is most polar. Answeredjul 24, 2019by anshuman sharma(78.3kpoints) selectedjul 25, 2019by kumari prachi. Br and i do not have sufficient electronegativity.
Also Read :





