The entropy of a substance decreases as its temperature increases. Entropy is the degree of disorderliness in a system.
Which Of The Following Has The Highest Entropy. 2 kg of hcn d. What element has the highest entropy? Which of the following statements concerning entropy is/are correct? So (b) co(g) has the highest standard entropy.
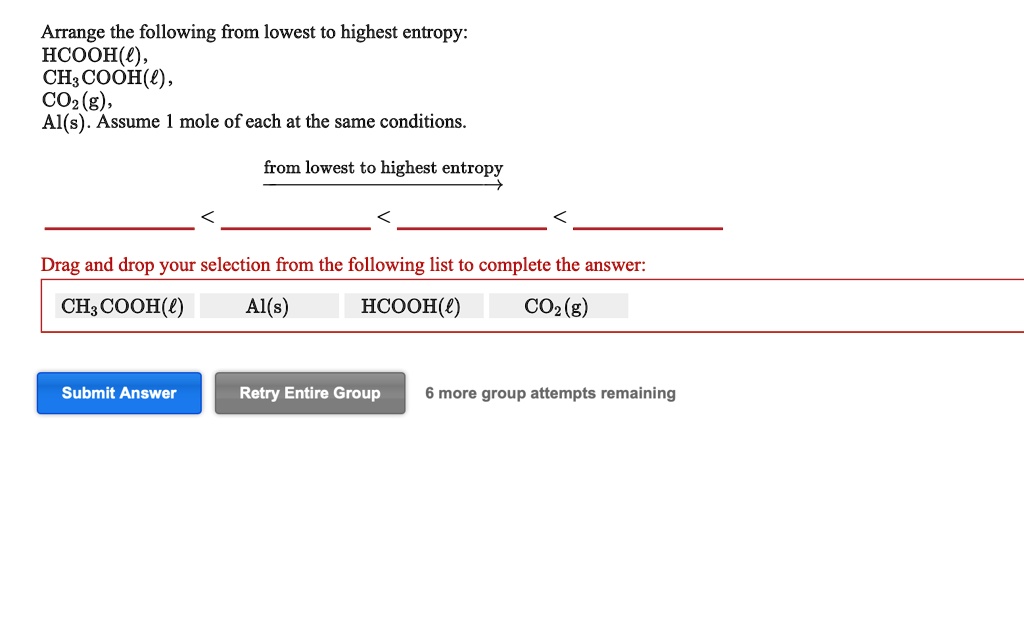 Solved:arrange The Following From Lowest To Highest Entropy: Hcooh(‚¬) , Ch: Cooh(E) , Co2 (G) , Al(S) . Assume Mole Of Each At The Same Conditions From Lowest To Highest Entropy Drag From numerade.com
Solved:arrange The Following From Lowest To Highest Entropy: Hcooh(‚¬) , Ch: Cooh(E) , Co2 (G) , Al(S) . Assume Mole Of Each At The Same Conditions From Lowest To Highest Entropy Drag From numerade.com
Related Post Solved:arrange The Following From Lowest To Highest Entropy: Hcooh(‚¬) , Ch: Cooh(E) , Co2 (G) , Al(S) . Assume Mole Of Each At The Same Conditions From Lowest To Highest Entropy Drag :
2 mol of hcn e. Indicate which of the following has the lowest standard molar entropy (s°). 1 mol of hcn c. So, its entropy will be highest.
For liquid state, still the particles are moving but less freely than the gas particles and more than the solid.
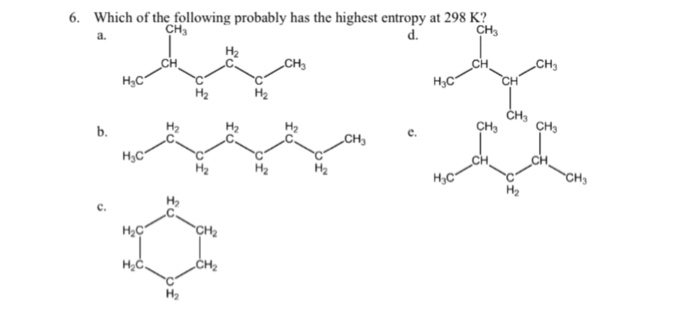
Liquids have more microstates (since the molecules can translate) and thus have a higher entropy. Among the phases, solid has the most ordered structure. 6) determine the entropy change of a system of 145 g of water when it is heated. Solid, liquid and gas, we can see that the gas particles move freely and therefore, the degree of randomness is the highest. Indicate which of the following has the highest entropy at 298 k. Here hydrogen gas has more entropy as it shows more randomness/disorderliness due to less molar mass than all the given substances and also in the gas phase.
 Source: chegg.com
Source: chegg.com
Asked sep 17, 2016 in chemistry by messi10. It is the only metal present as liquid at room temperature. 2 mol of hcn e.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
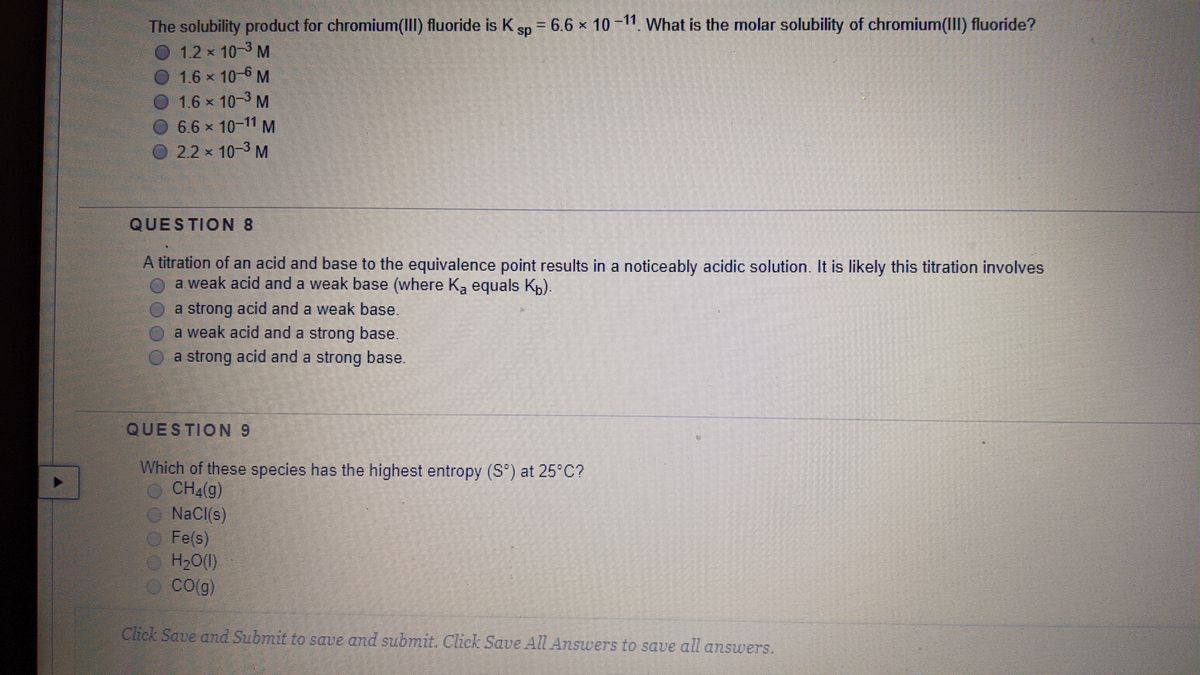
Hence, the correct option is (b). Which of the following has the largest standard molar entropy, s° (298.15 k)? (a) al (s) (b) caco₃ (s) (c) h₂o (i) (d) co₂ (g)
 Source: clutchprep.com
Source: clutchprep.com
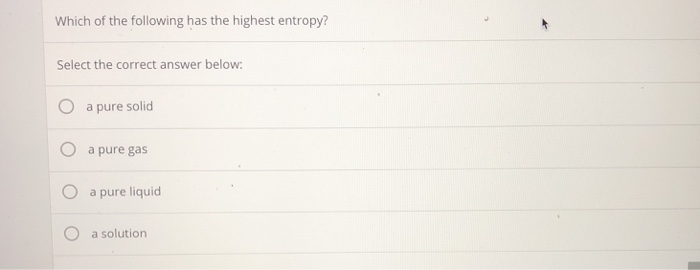
Which of the following has the highest standard molar entropy, s, at 25 c? If we look at the three states of matter: G as > l iq u id > s o lid
 Source: chegg.com
Source: chegg.com
Hence, entropy is maximum for water vapours. 2 so2 (g)+ o2 (g) → 2 so3 (g) 248.1 205.0 256.6. You may assume the enthalpy of fusion is independent of temperature.
 Source: clutchprep.com
Source: clutchprep.com
2 mg (s) + o₂ (g) → 2 mgo (s) Thus, in the last we can conclude that the one molecule of hydrogen gas has the highest entropy. All substances have positive standard molar entropies at temperatures above 0 k
 Source: chegg.com
Source: chegg.com
Hence, option b is correct. Which of the following has the largest standard molar entropy, s° (298.15 k)? But 02 has a lower molecular weight.
 Source: toppr.com
Source: toppr.com
Here hydrogen gas has more entropy as it shows more randomness/disorderliness due to less molar mass than all the given substances and also in the gas phase. The greater the disorder in a system, the higher is the entropy. It will be maximum in gases followed by liquids and least in solids.
 Source: bartleby.com
Source: bartleby.com
Here hydrogen gas has more entropy as it shows more randomness/disorderliness due to less molar mass than all the given substances and also in the gas phase. The measure of randomness of a substance is called entropy. Here hydrogen gas has more entropy as it shows more randomness/disorderliness due to less molar mass than all the given substances and also in the gas phase.
 Source: studylib.net
Source: studylib.net
Also, which has the greatest entropy? All substances have positive standard molar entropies at temperatures above 0 k What element has the highest entropy?
 Source: toppr.com
Source: toppr.com
Aieee , bank exams , cat , gate. Thus, in the last we can conclude that the one molecule of hydrogen gas has the highest entropy. So if we have a gram of each, so divide by 32 using our dimensional analysis, we saved that the amount of 02 is greater than that of 03 since we have the same mass.

Which of the following has the largest standard molar entropy, s° (298.15 k)? The more ways there are to distribute the molecules. Which of the following has the largest standard molar entropy, s° (298.15 k)?
 Source: numerade.com
Source: numerade.com
1 mol of hcn c. Which of the following forms of water has the highest entropy? Entropy is the degree of disorderliness in a system.
 Source: chegg.com
Source: chegg.com
2 kg of hcn d. Don’t get confused while determining the molecule with the higher entropy. Which one of the following has the highest standard molar entropy, s°, at 25°c?

It is the only metal present as liquid at room temperature. So if we have a gram of each, so divide by 32 using our dimensional analysis, we saved that the amount of 02 is greater than that of 03 since we have the same mass. Aieee , bank exams , cat , gate.
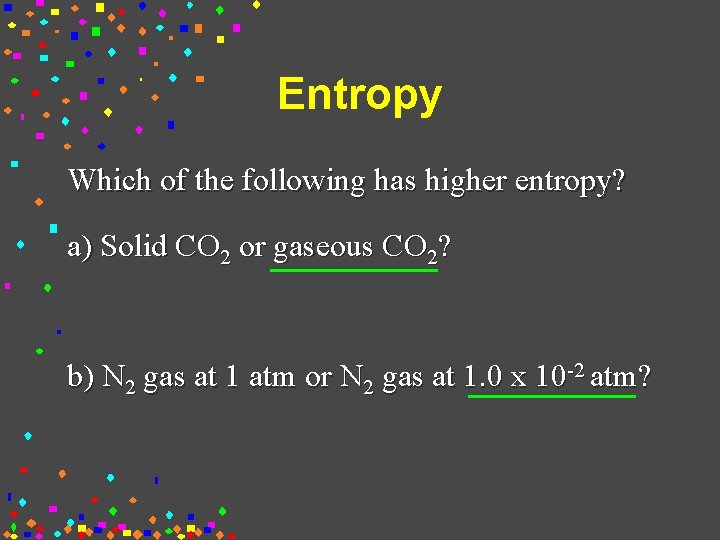 Source: slidetodoc.com
Source: slidetodoc.com
Which of the following has the highest standard molar entropy, s, at 25 c? (a) al (s) (b) caco₃ (s) (c) h₂o (i) (d) co₂ (g) The larger the volume the more ways there are to distribute the molecules in that volume;
 Source: doubtnut.com
Source: doubtnut.com
Which of the following has the highest standard molar entropy, s, at 25 c? It is the only metal present as liquid at room temperature. That said, nacl (g) would have the highest entropy.
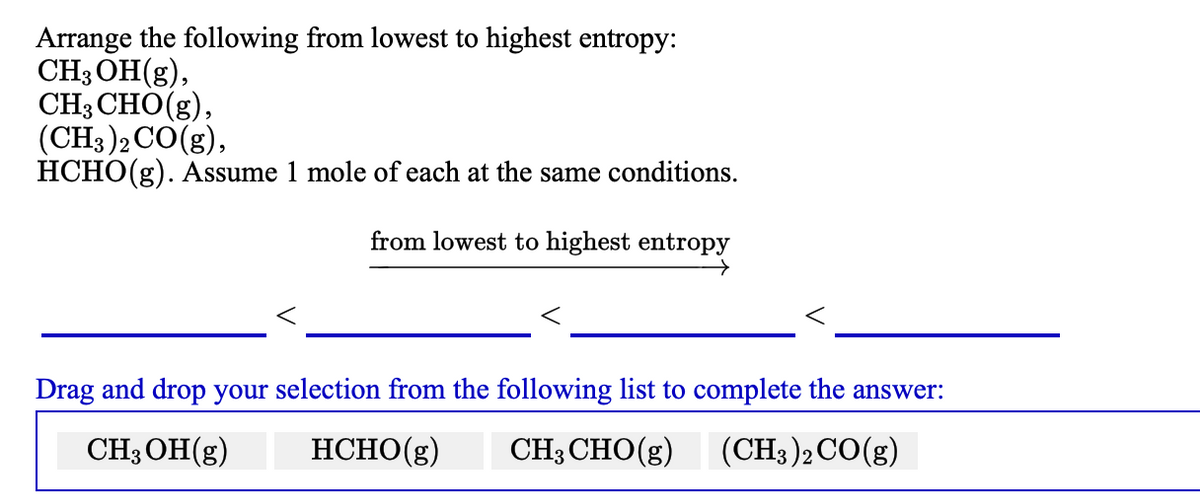 Source: bartleby.com
Source: bartleby.com
- determine the entropy change of a system of 145 g of water when it is heated. Entropy is a measure of disorder. 0.5 g of hcn b.
 Source: itprospt.com
Source: itprospt.com
G as > l iq u id > s o lid Soobee72pl and 3 more users found this answer helpful. (a) al (s) (b) caco₃ (s) (c) h₂o (i) (d) co₂ (g)
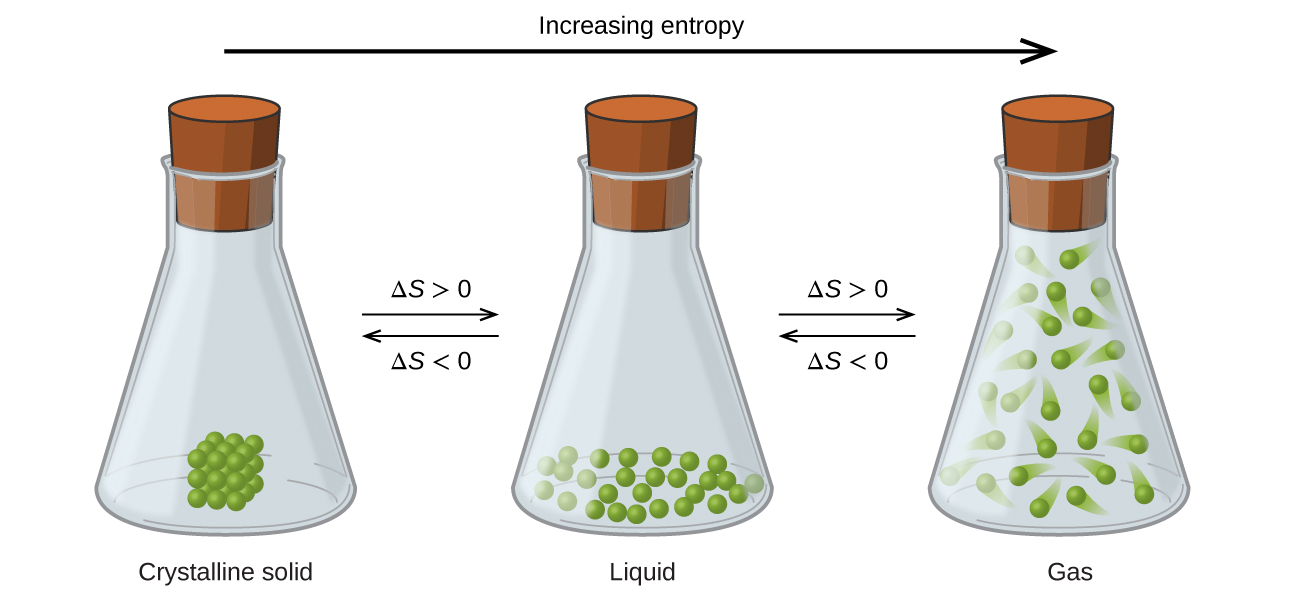 Source: socratic.org
Source: socratic.org
The larger the volume the more ways there are to distribute the molecules in that volume; Entropy is a measure of disorder. When a substance is a gas it has many more microstates and thus have the highest entropy.
 Source: clutchprep.com
Source: clutchprep.com
The larger the volume the more ways there are to distribute the molecules in that volume; Liquids have more microstates (since the molecules can translate) and thus have a higher entropy. For liquid state, still the particles are moving but less freely than the gas particles and more than the solid.
Also Read :





