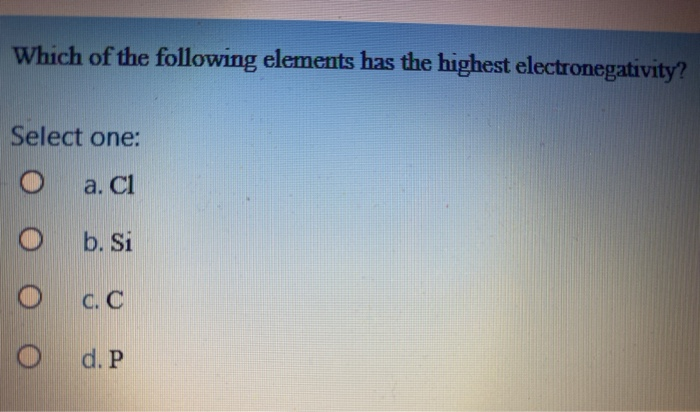
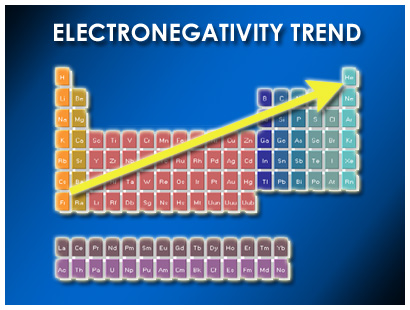
Which of the following atoms has the highest electronegativity? The most eletronegative elements are to the right of the periodic table.
Which Of The Following Has The Highest Electronegativity. Which of the following elements has the highest electronegativity? Annm88059 annm88059 06/14/2016 chemistry high school answered which of the following atoms has the highest electronegativity? (optional) use the periodic table to answer this question a. Of the following elements, which has the highest electronegativity?
 Solved Which Of The Following Elements Has The Highest | Chegg.com From chegg.com
Solved Which Of The Following Elements Has The Highest | Chegg.com From chegg.com
Related Post Solved Which Of The Following Elements Has The Highest | Chegg.com :
Fluorine is the most electronegative element in the world. W) lithium x) iodine y) cesium z) oxygen. Which of the following has the highest electronegativity: Element electronegativity br 2.8 p 2.1 mg 1.2 la 1.0 cs 0.7.
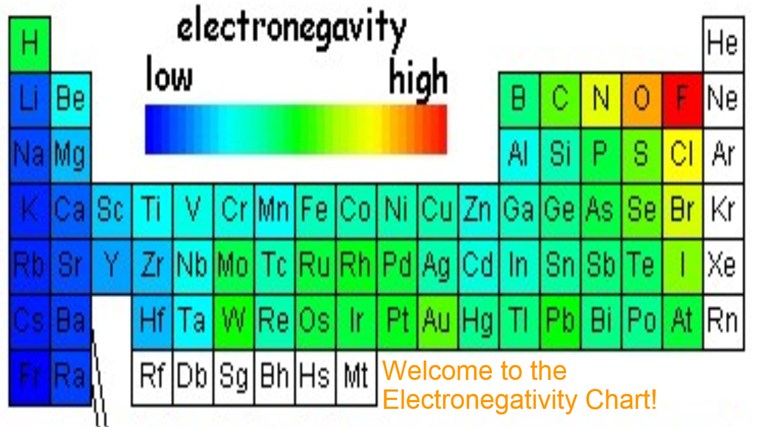
The most electronegative element is fluorine with a score of 4.0 (the highest possible.) across from fluorine we also have n and o with high electronegativities.
Of the following elements, which has the highest electronegativity? Of the following elements, which has the highest electronegativity? Which is more electronegative f or f? Out of following which has the highest electronegativity ? (i) element forms a carbonate which is not decomposed by heating (ii) element is most likely to form coloured ionic compounds (iii) element has largest atomic radius (iv) element forms only acidic oxide Elements from the halogen group including f, cl, br have pretty high electronegativities.

One may also ask, which alkali metal has the highest electronegativity? 117 rows the electronegativity chart describes how atoms can attract a pair of. Thus, fluorine is the most electronegative element, while francium is one of the least electronegative.
 Source: periodictable.me
Source: periodictable.me
That is why h2o is a polar molecule with more electron density on the o and less on the h. The stable electron configuration of the 2 p orbital contains 6 electrons. Ncert dc pandey sunil batra hc verma pradeep errorless.
 Source: chegg.com
Source: chegg.com
Electronegativity is basically how much elements �want� electrons. Also asked, which element has the highest electronegativity and why? As fluorine is very close to ideal electronic configuration, hence the electrons are held very tightly to the nucleus.
 Source: chegg.com
Source: chegg.com
Based on these electronegativities, sih4 would be expected to. Compound bond strength, kj/mol hf 569.87 hcl 431.62 hbr 366.35 hi 298.407 briefly explain why the bond strengths are ranked in this order. Elements with high electronegativity will attract shared electrons in a covalent bond more strongly.
 Source: angelo.edu
Source: angelo.edu
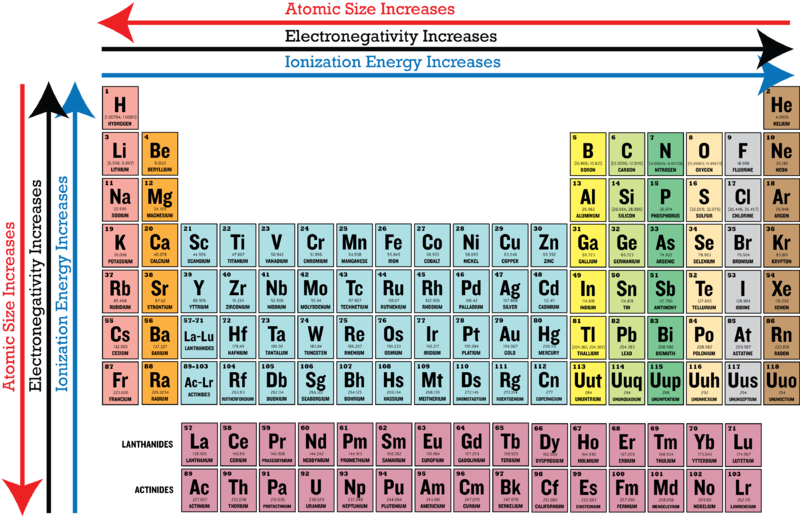
Electronegativity increases across a period, an horizontal row of the periodic table from left to right as we face the table, and decreases down a group, a column of the periodic table. Which statement best describes the bonding in a3b? What 3 elements have the highest electronegativity?
 Source: bartleby.com
Source: bartleby.com
Chlorine is the most electronegative element out of the possible choices. Elements from the halogen group including f, cl, br have pretty high electronegativities. Also asked, which element has the highest electronegativity and why?
 Source: slideplayer.com
Source: slideplayer.com
Similarly in the case of o—r, o is more electronegative than r where “r” is an alkyl group. Determine which sequence of elements satisfy the following statements: Electronegativity increases from bottom to top in groups, and increases from left to right across periods.
 Source: vedantu.com
Source: vedantu.com
(optional) use the periodic table to answer this question a. To see more answers head over to college study guides. Based on the indicated electronegativities, arrange the following in order of increasing ionic character:
 Source: slideplayer.com
Source: slideplayer.com
Fluorineof the main group elements, fluorine has the highest electronegativity (en = 4.0) and cesium the lowest (en = 0.79). Similarly in the case of o—r, o is more electronegative than r where “r” is an alkyl group. To keep reading this solution, download the app.
 Source: secrets-of-periodic-table.blogspot.com
Source: secrets-of-periodic-table.blogspot.com
As fluorine is very close to ideal electronic configuration, hence the electrons are held very tightly to the nucleus. Therefore, chlorine has a higher electron affinity than fluorine. Element a has an electronegativity of 0.8 and element b has an electronegativity of 3.0.
 Source: chegg.com
Source: chegg.com
Therefore, chlorine has a higher electron affinity than fluorine. The most eletronegative elements are to the right of the periodic table. Which is more electronegative f or f?
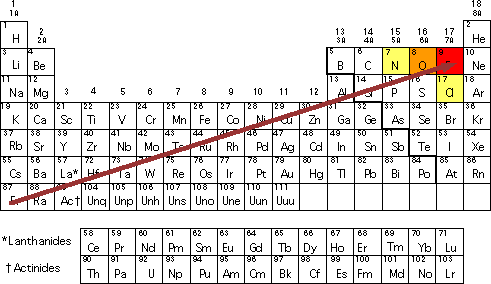 Source: westfield.ma.edu
Source: westfield.ma.edu
Chlorine is the most electronegative element out of the possible choices. Element electronegativity br 2.8 p 2.1 mg 1.2 la 1.0 cs 0.7. Therefore, chlorine has a higher electron affinity than fluorine.
 Source: slideplayer.com
Source: slideplayer.com
In the periodic table the electronegativities range from 0.7 for cesium, the least electronegative of the elements, to 4.0 for fluorine, the most electronegative. Electronegativity is the ability of atoms to attract electrons in a covalent bond. Based on these electronegativities, sih4 would be expected to.
 Source: thoughtco.com
Source: thoughtco.com
Which of the following atoms has the highest electronegativity? Which of the following elements has the highest electronegativity? To see more answers head over to college study guides.
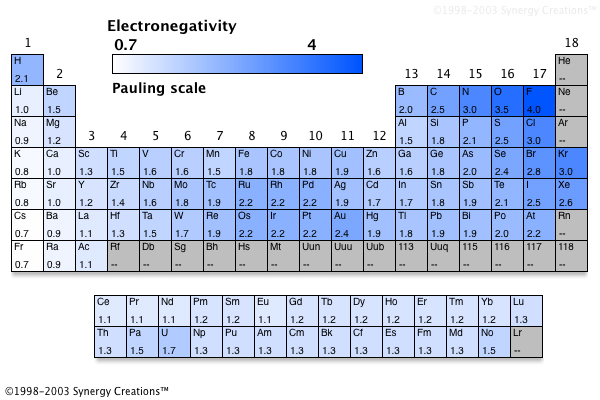 Source: socratic.org
Source: socratic.org
Second, neither oh nor or pulls the bonding electrons to itself. Based on these electronegativities, sih4 would be expected to. The two elements required to form substitutional solid solution should not have (a) same crystalline structure (b) same valency (c) widely differing.
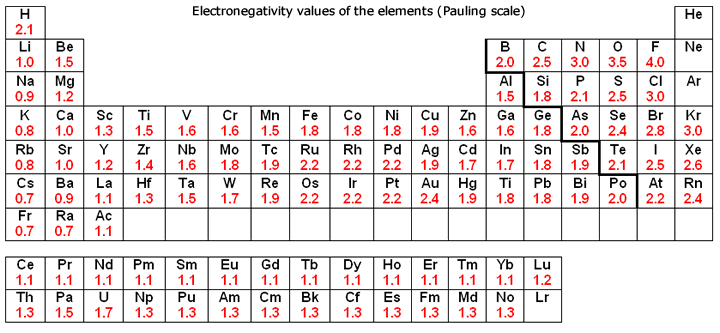 Source: socratic.org
Source: socratic.org
Based on these electronegativities, sih4 would be expected to. Similarly in the case of o—r, o is more electronegative than r where “r” is an alkyl group. Generally (though not 100% of the time) electronegativity increases as you go further left and up the periodic table.

Of the following elements, which has the highest electronegativity? As b.p o c.s d. Electronegativity is the ability of atoms to attract electrons in a covalent bond.
 Source: youtube.com
Source: youtube.com
Also asked, which element has the highest electronegativity and why? The most electronegative element is fluorine with a score of 4.0 (the highest possible.) across from fluorine we also have n and o with high electronegativities. Electronegativity is basically how much elements �want� electrons.
 Source: angelo.edu
Source: angelo.edu
Which of the following elements has the highest electronegativity? Based on the indicated electronegativities, arrange the following in order of increasing ionic character: Elements from the halogen group including f, cl, br have pretty high electronegativities.
 Source: ontrack-media.net
Source: ontrack-media.net
(optional) use the periodic table to answer th… get the answers you need, now! Which statement best describes the bonding in a3b? Asked jan 11 in chemistry by chetandivakar (22.5k points) which of the following elements has the highest electronegativity ?
Also Read :





