Examples include hydrogen (h), lithium (li), and sodium (na). The atoms' valence electrons are shared between.
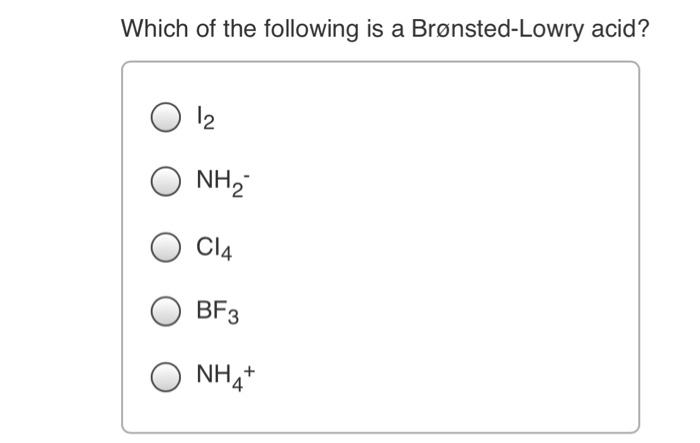
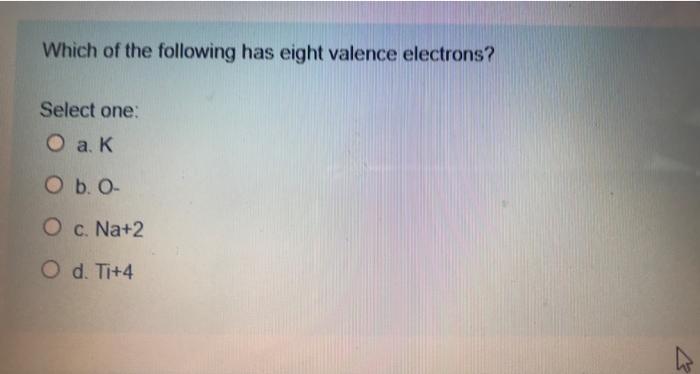
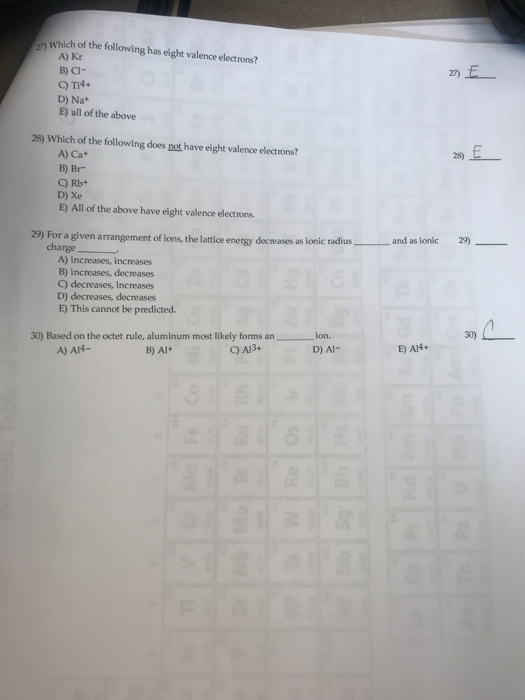
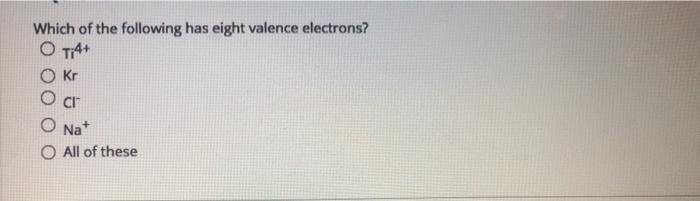
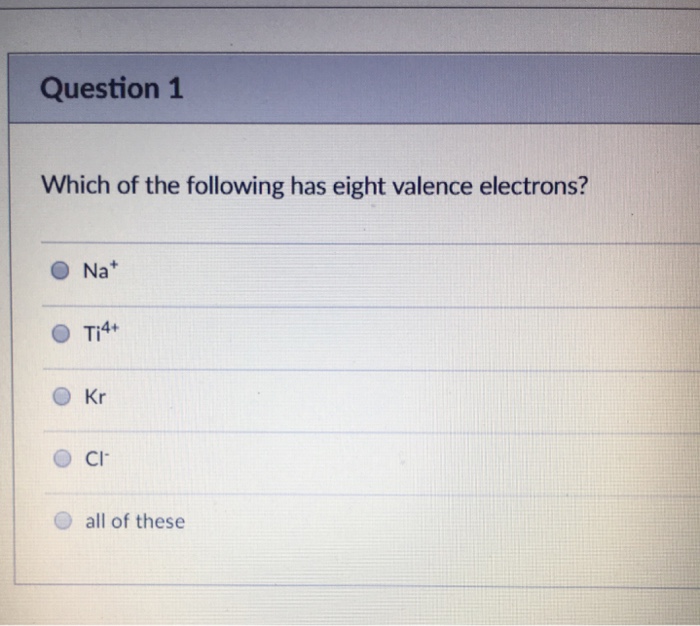
Which Of The Following Has Eight Valence Electrons. Hence, ca+ does not have 8 electrons in its valence shell. Which of the following has eight valence electrons? 10 in the lewis symbol for a sulfur atom, there are _____ paired and _____ unpaired electrons. The only way for magnesium to accomplish this is to give up its two valence electrons and have one of its filled energy levels become the valence shell, i.e.
 Valence Electrons - Msrblog From msrblog.com
Valence Electrons - Msrblog From msrblog.com
Related Post Valence Electrons - Msrblog :
Atoms tend to gain, share, lose valence electrons until each atom has the same number of valence electrons as the _____. These questions are based on the attached photo. To attain 8 electrons in its valence shell, it should lose two electrons from its valence shell and attain the configuration [he] 2s2 2p6 3s2 3p6. Which of the following has eight valence electrons?
3s2 3p6 total number of valence electrons = 8.
Atoms with 8 electrons in their valence shell have completely filled last orbitals and are therefore the most stable, as their electronic configuration is similar to that of the closest noble gas. The outermost orbit of the atom cannot have less than 8 electrons. This valence electron participates in the formation of bonds with atoms of other elements. The number 8 is a lucky number, according to many asian cultures. Hence, ca+ does not have 8 electrons in its valence shell. It has only one electron in its valence shell.

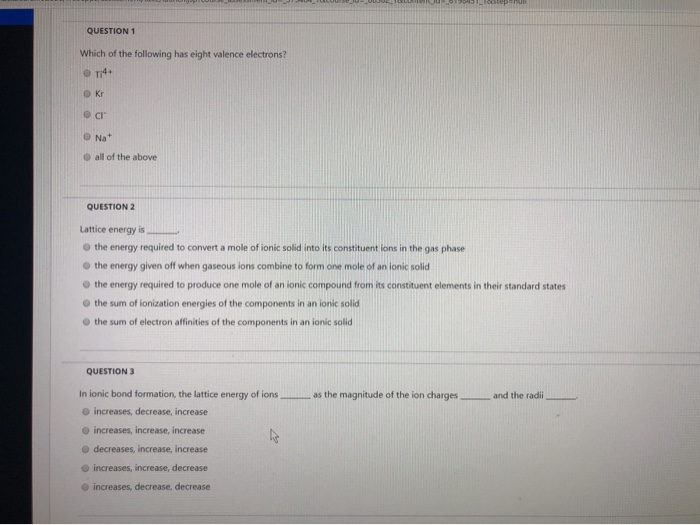
The atoms� valence electrons are shared between. The electron configuration of neon(ne) shows that the last shell of neon has eight electrons. 3) lattice energy is _____.
Therefore, the valence electrons of neon(ne) are eight. 3) lattice energy is _____. 3s2 3p6 total number of valence electrons = 8.
 Source: msrblog.com
Source: msrblog.com
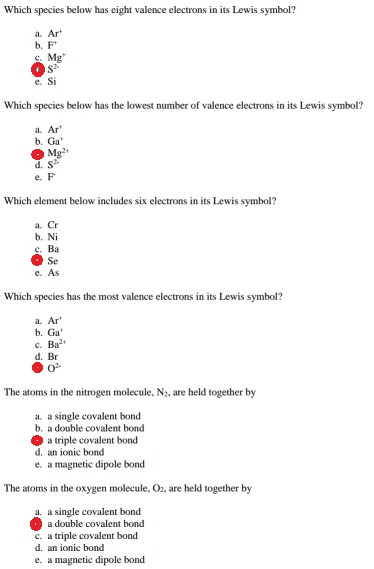
1 show answers another question on chemistry. The columns that were set up to group elements by similar chemical properties turn out to be the exact same columns defined by the number of valence electrons. Oxygen participates in the formation of bonds through its valence electrons.

Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). The number 8 is a lucky number, according to many asian cultures. This valence electron participates in the formation of bonds with atoms of other elements.
 Source: chegg.com
Source: chegg.com
The atoms� valence electrons are shared between. Which of the following has eight valence electrons? D) the oxygen no longer has eight valence electrons.
 Source: itprospt.com
Source: itprospt.com
The number of electrons per shell of phosphorus is 2, 8, 5. The outermost orbit of the atom cannot have less than 8 electrons. Examples include neon (ne), argon (ar), and krypton (kr).
 Source: chegg.com
Source: chegg.com
A) both bonding electrons come from the oxygen atom. The atoms� valence electrons are shared between. If an orbit has 8 electrons, then it will be the outermost orbit of that atom.
 Source: chegg.com
Source: chegg.com
Any element in group 18 has eight valence electrons (except for helium, which has a total of just two electrons). Examples include hydrogen (h), lithium (li), and sodium (na). Valence electrons are transferred from one atom to the other.
Examples include hydrogen (h), lithium (li), and sodium (na). Examples include neon (ne), argon (ar), and krypton (kr). Hence, ca+ does not have 8 electrons in its valence shell.
 Source: chegg.com
Source: chegg.com
Atoms tend to gain, share, lose valence electrons until each atom has the same number of valence electrons as the _____. Valence electrons are transferred from one atom to the other. Hence, the central atom, i.e., p has more than 8 electrons in its outer shell and octet of oxygen is completed.

Atoms with 8 electrons in their valence shell have completely filled last orbitals and are therefore the most stable, as their electronic configuration is similar to that of the closest noble gas. Configuration of the valence shell: Cl is right next to argon.
 Source: oneclass.com
Source: oneclass.com
- lattice energy is _____. Electrons in the outermost shell are called valence electrons, because it is their interactions that determine the chemical properties of an element. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
The electron configuration of neon(ne) shows that the last shell of neon has eight electrons. H2o to form the hydronium ion h3o+, this bond is called a coordinate covalent bond because ____. Electrons in the outermost shell are called valence electrons, because it is their interactions that determine the chemical properties of an element.
 Source: chegg.com
Source: chegg.com
H2o to form the hydronium ion h3o+, this bond is called a coordinate covalent bond because ____. Therefore, the valence electrons of neon(ne) are eight. Helium has two electrons in its outermost shell and all other elements have atoms with eight electrons in the outermost shell.
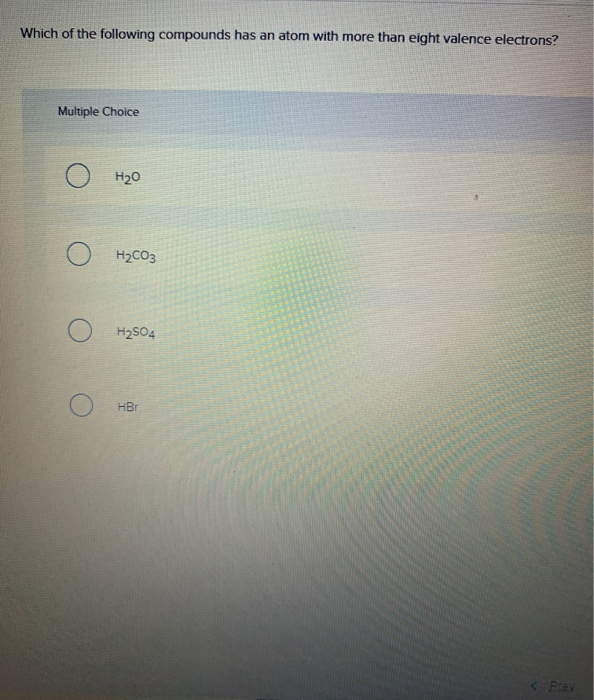 Source: chegg.com
Source: chegg.com
Helium has two electrons in its outermost shell and all other elements have atoms with eight electrons in the outermost shell. A) both bonding electrons come from the oxygen atom. This valence electron participates in the formation of bonds with atoms of other elements.
 Source: khanacademy.org
Source: khanacademy.org
But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core. Helium has two electrons in its outermost shell and all other elements have atoms with eight electrons in the outermost shell. E) all of the above.
 Source: chegg.com
Source: chegg.com
All there are ____ valence electrons in the lewis structure of ch_3, ch_2cl. If an orbit has 8 electrons, then it will be the outermost orbit of that atom. Configuration of the valence shell:
 Source: khanacademy.org
Source: khanacademy.org
Anything that is on the far right hand side of the periodic table has eight valence electrons. Oxygen participates in the formation of bonds through its valence electrons. The last shell after the electron configuration is called the valence shell (orbit).
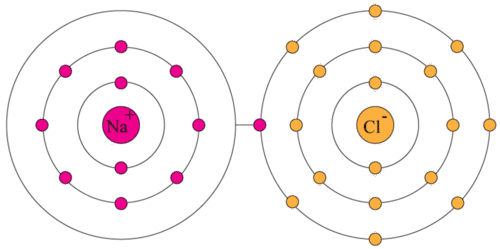
If an orbit has 8 electrons, then it will be the outermost orbit of that atom. The only way for magnesium to accomplish this is to give up its two valence electrons and have one of its filled energy levels become the valence shell, i.e. 10 in the lewis symbol for a sulfur atom, there are _____ paired and _____ unpaired electrons.

To attain 8 electrons in its valence shell, it should lose two electrons from its valence shell and attain the configuration [he] 2s2 2p6 3s2 3p6. 2) which of the following does not have eight valence electrons? In order to complete its octet, it must get to 8 valence electrons.
Also Read :