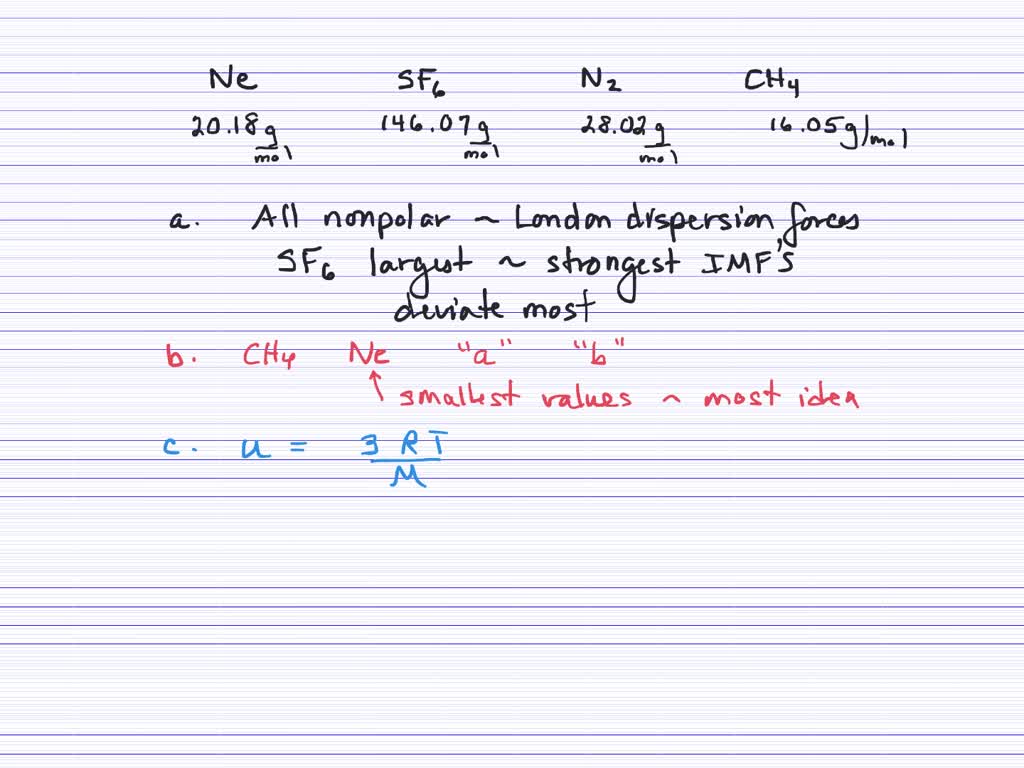
The gas with the lowest molecular weight will effuse the fastest. Which of the following gases will effuse most slowly under the same physical conditions?
Which Of The Following Gases Will Effuse The Most Rapidly. Hydrogen will effuse most rapidly because it has the least molar mass among the gases given. Are usually the most abundant gases emitted during basaltic volcanism; The heavier a gas is, the slower it will effuse. ____12) which gas diffuses most rapidly at stp?
 Gas Laws. - Ppt Video Online Download From slideplayer.com
Gas Laws. - Ppt Video Online Download From slideplayer.com
Related Post Gas Laws. - Ppt Video Online Download :
Which gas is most likely to depart from assumption 3 of the kinetic molecular theory (attractive and repulsive forces between gas molecules are negligible.)? The lightest, and therefore fastest, gas is helium. Well, we know that effusion. Hydrogen will effuse most rapidly because it has the least molar mass among the gases given.
Are usually the most abundant gases emitted during basaltic volcanism;
Which of the following gases deviates most from ideal behavior; Which gas will diffuse most rapidly under the same conditions of temperature and pressure? 15)which of the following gases will effuse the most rapidly? A look at the periodic table shows that helium will diffuse faster. The heavier a gas is, the slower it will effuse. Chapter 14 review which of the following gases will effuse the most rapidly?
 Source: slideserve.com
Source: slideserve.com
Use graham’s law to calculate fast o 2 gas will effuse compared to cl 2. The lightest, and therefore fastest, gas is helium. So we have these four gases.
 Source: numerade.com
Source: numerade.com
Rate 1 rate 2 = √ molecular mass of gas 2 molecular mass of gas 1. Are usually the most abundant gases emitted during basaltic volcanism; Which gas would diffuse most quickly?
 Source: numerade.com
Source: numerade.com
Which of the following gases will effuse the most rapidly? The gas with the lowest molecular weight will effuse the fastest. So, ammonia gas diffuses a bit less than 1.5 times as fast as hydrogen chloride gas.
 Source: slideplayer.com
Source: slideplayer.com
Gas density & graham�s law (a) ne 13) calculate the densities of the following gases at stp: B) a mixture of gases contains 0.75mol n2, 0.35mol o2, and 0.20mol co2. Use graham’s law to calculate fast o 2 gas will effuse compared to cl 2.
 Source: chegg.com
Source: chegg.com
Which gas diffuses most rapidly? And the first question wants to know which gas will refuse the fastest. Which of the following will diffuse more quickly?
 Source: numerade.com
Source: numerade.com
Which gas diffuses most rapidly? But since density is proportional to the molecular mass. Of all the choices, helium (he) has the lowest molecular weight (atomic weight in this case), so it will have the highest rate of effusion.
 Source: chegg.com
Source: chegg.com
Which of the following will diffuse more quickly? Chapter 14 review which of the following gases will effuse the most rapidly? Rate 1 rate 2 = √ molecular mass of gas 2 molecular mass of gas 1.
 Source: studylib.net
Source: studylib.net
The rate of effusion is inversely proportional to the molar mass. Science chemistry q&a library which of the following gases will effuse most rapidly (fastest)? Don�t forget to check for diatomics!
 Source: coursehero.com
Source: coursehero.com
This can be more formally derived from kinetic. Which of the following gases will effuse most slowly under the same physical conditions? ____12) which gas diffuses most rapidly at stp?
 Source: chegg.com
Source: chegg.com
Hydrogen will effuse most rapidly because it has the least molar mass among the gases given. So we have these four gases. A)consider the following gases, all at stp:
 Source: slidetodoc.com
Source: slidetodoc.com
Under what conditions of temperature and pressure is the behavior of a real gas most like that of an ideal gas? The smaller the molar mass, the faster diffuses. Which of the following gases will effuse most rapidly?
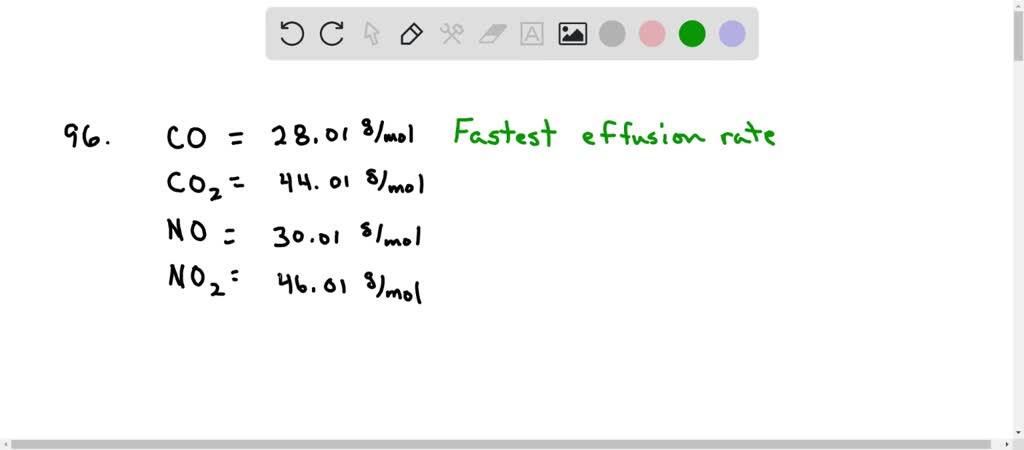 Source: numerade.com
Source: numerade.com
Well, we know that effusion. And the first question wants to know which gas will refuse the fastest. Which one would effuse more rapidly than n2?
 Source: youtube.com
Source: youtube.com
A)consider the following gases, all at stp: Which gas would diffuse most quickly? Use graham’s law to calculate fast o 2 gas will effuse compared to cl 2.
 Source: youtube.com
Source: youtube.com
And the first question wants to know which gas will refuse the fastest. Cl2 c.none of the above Which gas will diffuse most rapidly under the same conditions of temperature and pressure?

How many times faster is ammonia than hydrochloric acid? The rate of effusion is inversely proportional to the molar mass. B) a mixture of gases contains 0.75mol n2, 0.35mol o2, and 0.20mol co2.
 Source: chegg.com
Source: chegg.com
Which of the following gases will effuse the most rapidly? Which gas will diffuse most rapidly under the same conditions of temperature and pressure? Which gas is most likely to depart from assumption 3 of the kinetic molecular theory (attractive and repulsive forces between gas molecules are negligible.)?
 Source: youtube.com
Source: youtube.com
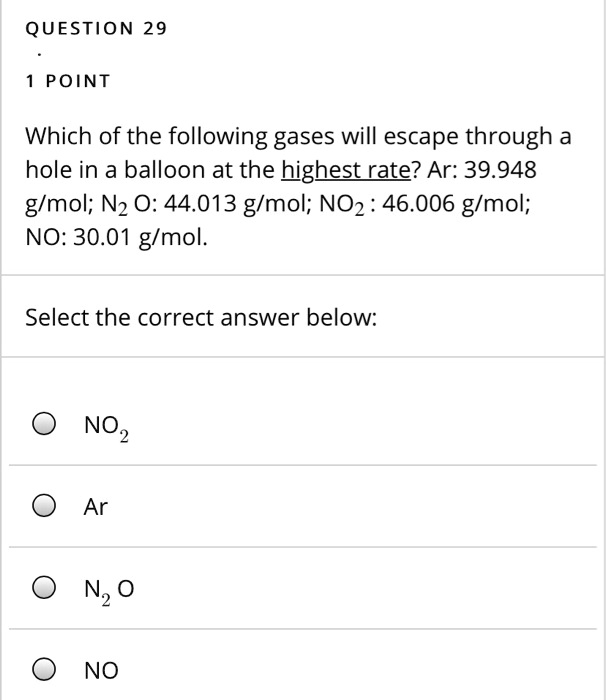
Which of the following gases will effuse most rapidly? The rate of effusion is inversely proportional to the molar mass. Which of the following gases effuses most rapidly?(a) nitrogen(b) oxygen(c) hydrogen chloride(d) ammonia(e) carbon monoxide
The rate of effusion is inversely proportional to the molar mass. Which of the following gases will effuse the most rapidly? Which one would effuse more rapidly than n2?
 Source: slideplayer.com
Source: slideplayer.com
Cl2 c.none of the above Under what conditions of temperature and pressure is the behavior of a real gas most like that of an ideal gas? So, ammonia gas diffuses a bit less than 1.5 times as fast as hydrogen chloride gas.
 Source: slideplayer.com
Source: slideplayer.com
Chapter 14 review which of the following gases will effuse the most rapidly? The heavier a gas is, the slower it will effuse. B) a mixture of gases contains 0.75mol n2, 0.35mol o2, and 0.20mol co2.
Also Read :