The allen scale assigns the lowest electronegativity to cesium, with a value of 0.659. The electronegativity of calcium (ca) is 1.
Which Of The Following Elements Has The Lowest Electronegativity. Hence, option d is correct. A measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound is called. Which element has the lowest electronegativity? Lithium, beryllium, magnesium, sodium 2.
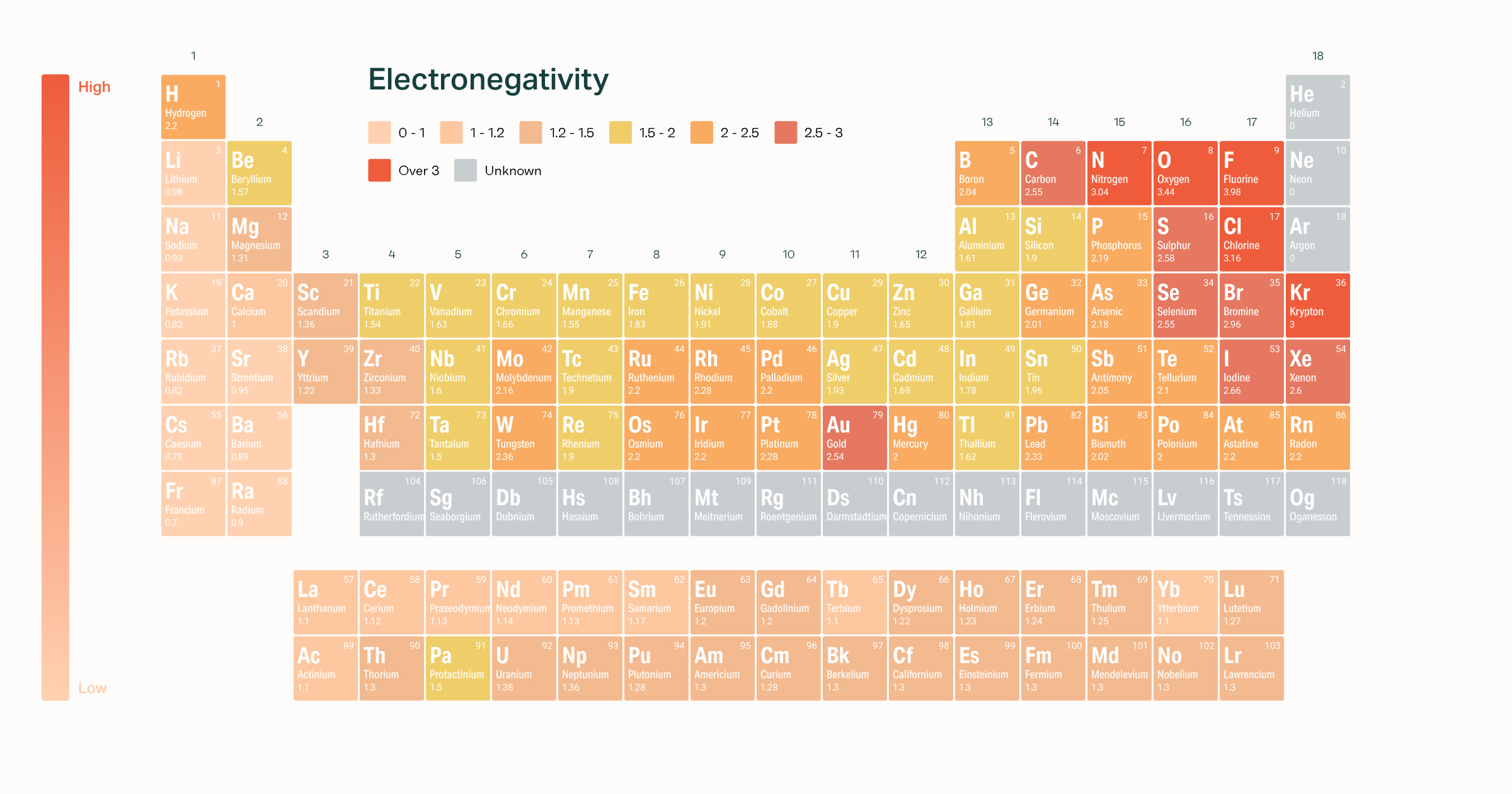 Electronegativity Of The Elements From breakingatom.com
Electronegativity Of The Elements From breakingatom.com
Related Post Electronegativity Of The Elements :
The alkali metals as a group have the lowest electronegativities, with the values falling as the atomic number increases, so the winner is caesium (cesium), with a pauling score of 0.79. This value uses the pauling scale to measure electronegativity. Fluorineof the main group elements, fluorine has the highest electronegativity (en = 4.0) and cesium the lowest (en = 0.79). The lowest electronegativity of the element from the following atomic number is.
They have few valence electrons and aren’t close to having eight valence electrons
Electronegativity increases from bottom to top in groups, and increases from left to right across periods. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least electronegative at 0.7. Among the given elements oxygen is more electronegative. A) 1 c) b) 6 d) 2 7. Which element has the lowest electronegativity? So such an element is fluorine in the given option whose electronegativity is 4.0.

Thus, fluorine is the most electronegative element, while francium is one of the least electronegative. The electronegativity of calcium (ca) is 1. Which out of the following elements has the least metallic character:
 Source: toppr.com
Source: toppr.com
Atoms with a low electronegativity, like lithium, have a weak attractive force for electrons because? It has 6 electrons in its outermost shell hence it needs 2 electrons to complete its octet so it will accept 2 electrons. Fluorineof the main group elements, fluorine has the highest electronegativity (en = 4.0) and cesium the lowest (en = 0.79).
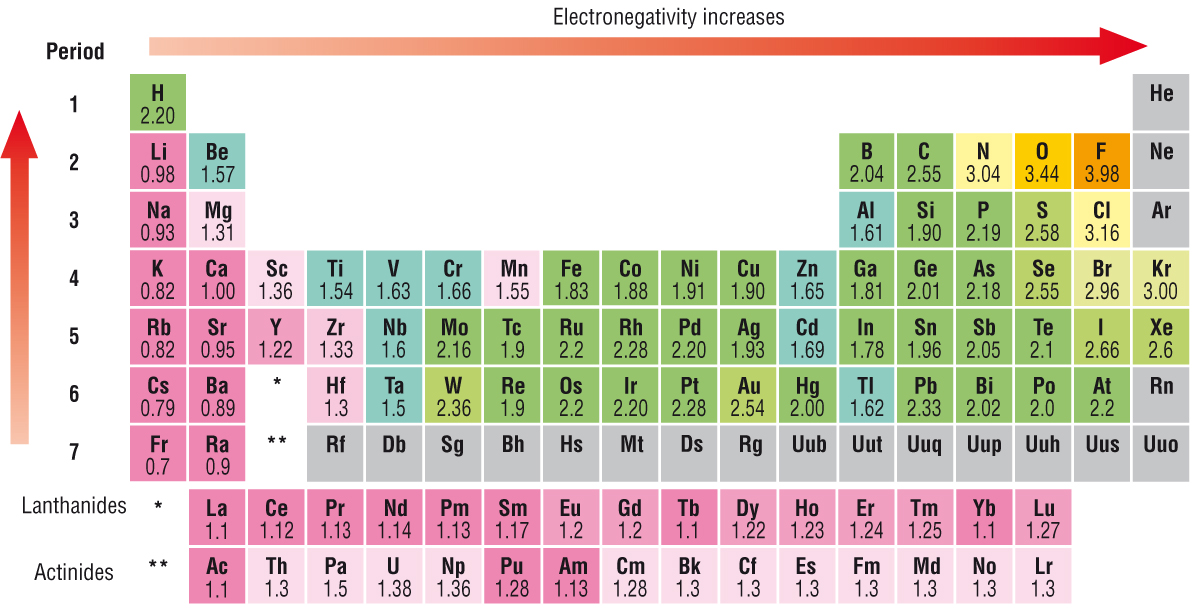 Source: socratic.org
Source: socratic.org
Thus, fluorine is the most electronegative element, while francium is one of the least electronegative. What are the name and group number of the chemical family that has the lowest overall electronegativities? It has 6 electrons in its outermost shell hence it needs 2 electrons to complete its octet so it will accept 2 electrons.
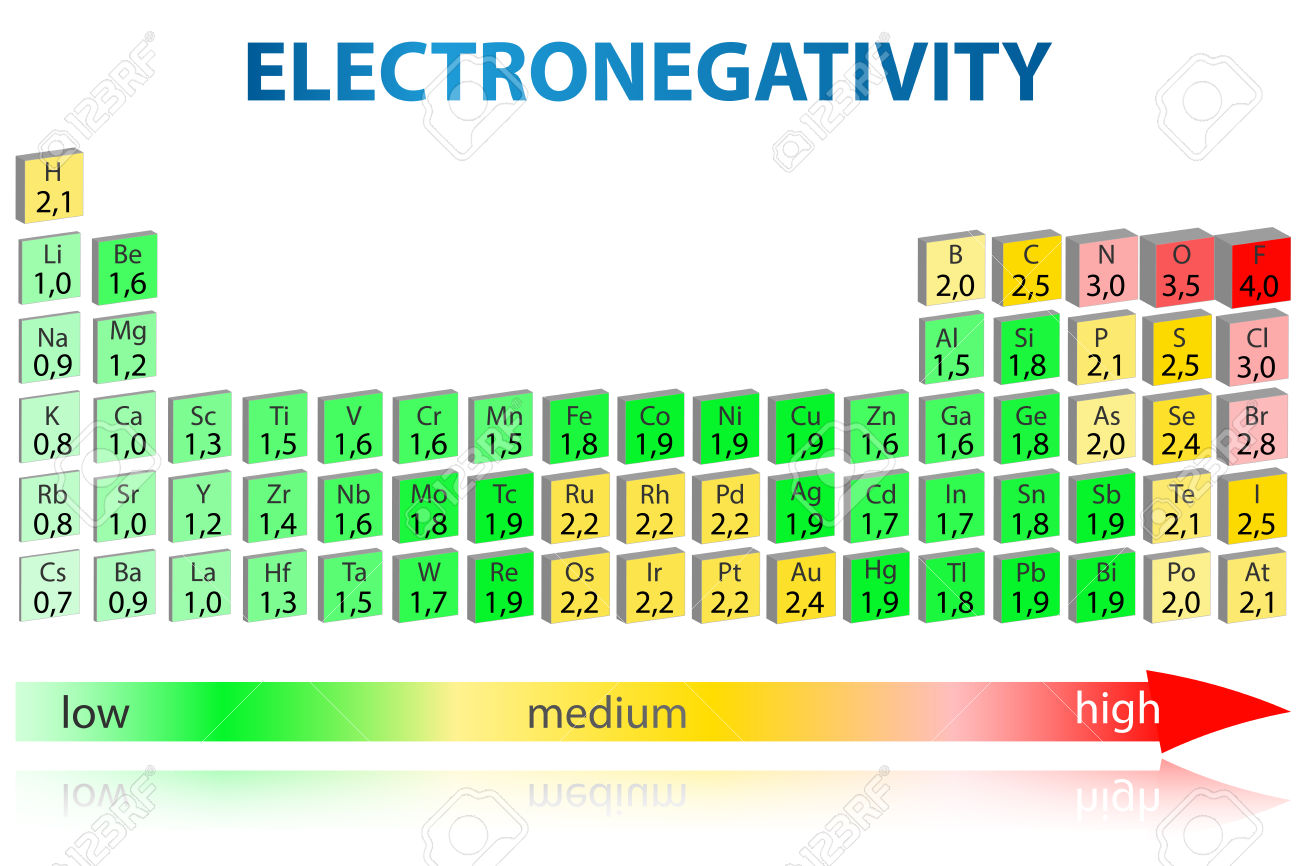 Source: socratic.org
Source: socratic.org
Choice calcium (ca) nitrogen (n) silicon (si) rubidium (rb) 10 10a) which of the following elements has the highest electronegativity? Fluorineof the main group elements, fluorine has the highest electronegativity (en = 4.0) and cesium the lowest (en = 0.79). The electronegativity of magnseium (mg) is 1.31 (has the highest) the electronegativity of barium (ba) is 0.89.
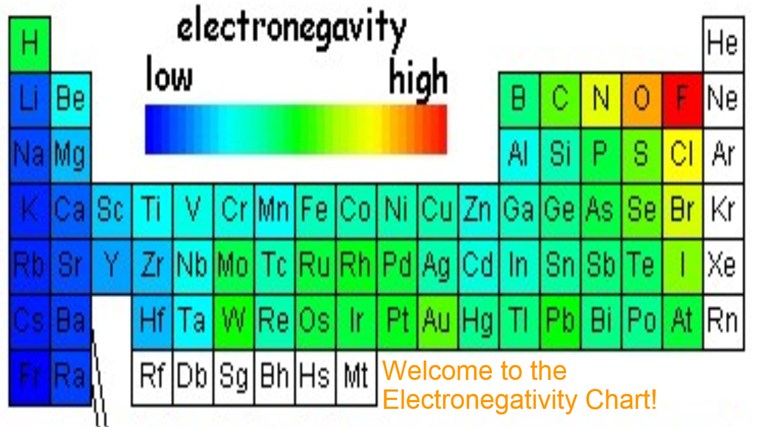 Source: periodictable.me
Source: periodictable.me
Fluorineof the main group elements, fluorine has the highest electronegativity (en = 4.0) and cesium the lowest (en = 0.79). Electronegativity increases from bottom to top in groups, and increases from left to right across periods. Group 18 elements have no electronegativity.
 Source: quizlet.com
Source: quizlet.com
Thus, fluorine is the most electronegative element, while francium is one of the least electronegative. What are the name and group number of the chemical family that has the lowest overall electronegativities? Which element has the lowest electronegativity?
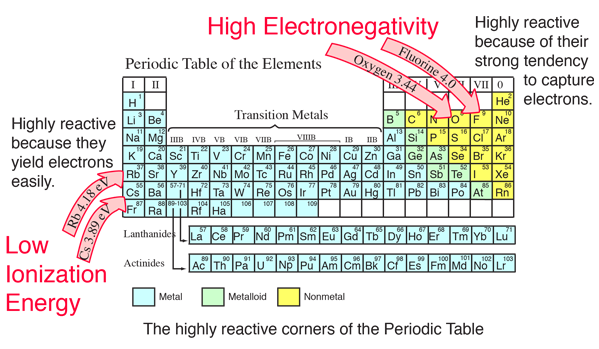 Source: hyperphysics.phy-astr.gsu.edu
Source: hyperphysics.phy-astr.gsu.edu
It has the atomic number of 55. What are the name and group number of the chemical family that has the lowest overall electronegativities? This value uses the pauling scale to measure electronegativity.
Which element has the lowest electronegativity? A) sodium c) aluminum b) potassium d) phosphorus. What is the numerical value?
 Source: slideplayer.com
Source: slideplayer.com
So, iodine has the lowest electronegativity. As such, it has a lower tendency to attract electrons , and thus has a lower electronegativity. B beryllium is a metal, and metals have higher values of electronegativity than nonmetals of the same period.;
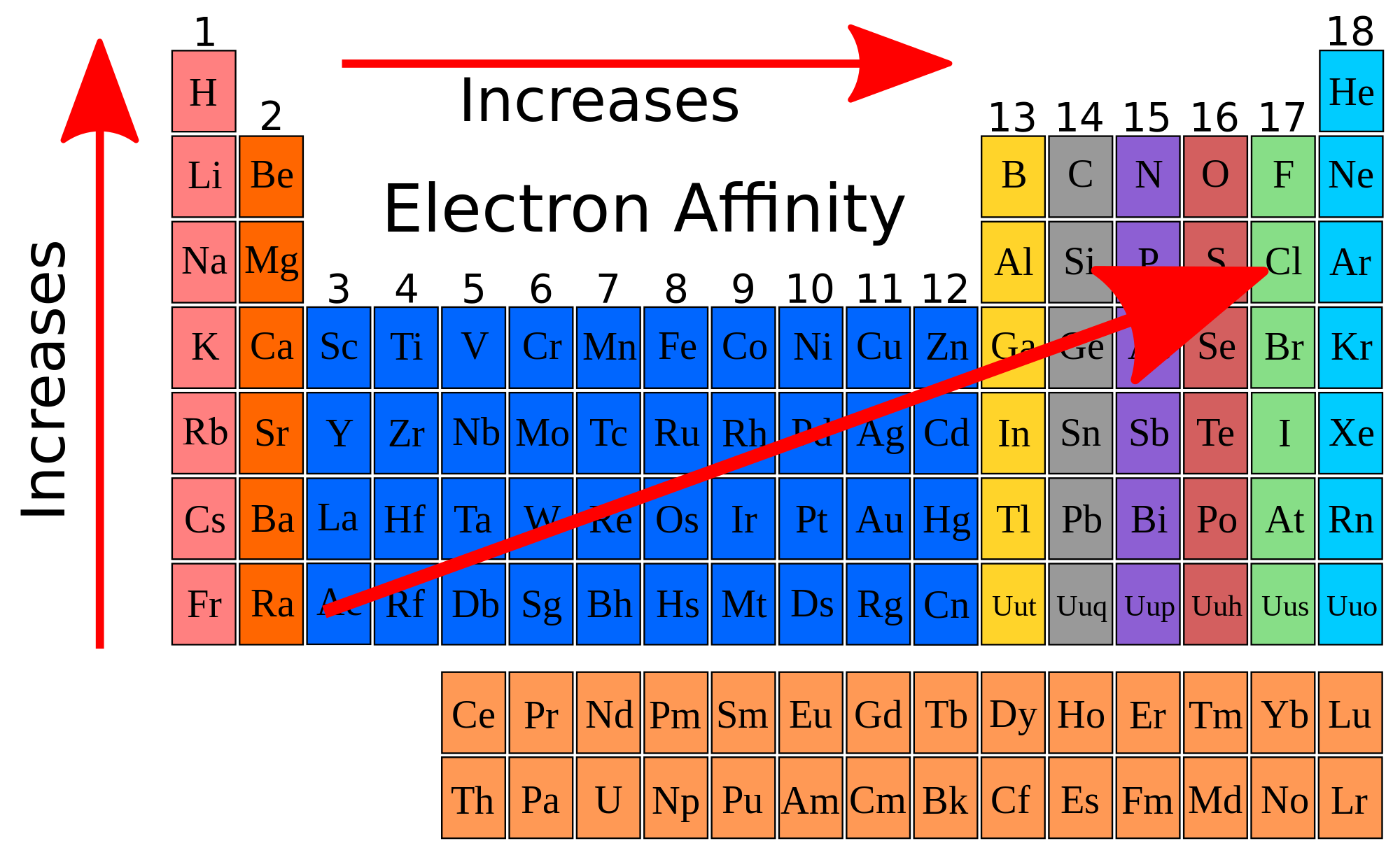 Source: socratic.org
Source: socratic.org
Element electronegativity br 2.8 p 2.1 mg 1.2 la 1.0 cs 0.7 Fluorineof the main group elements, fluorine has the highest electronegativity (en = 4.0) and cesium the lowest (en = 0.79). As such, it has a lower tendency to attract electrons , and thus has a lower electronegativity.
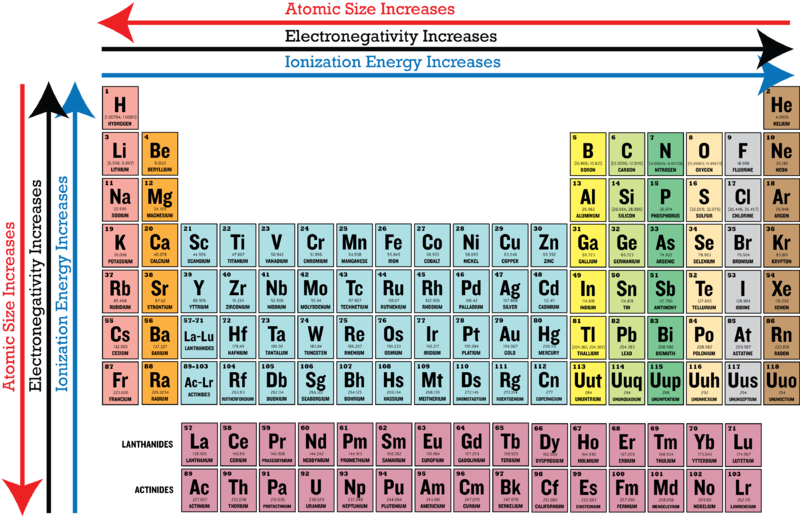 Source: vedantu.com
Source: vedantu.com
The element with the lowest electronegativity value is francium, which has an electronegativity of 0.7. It has the atomic number of 17. Hence, option d is correct.
 Source: slideplayer.com
Source: slideplayer.com
This is because they already have eight electrons in their outermost shell and. Element electronegativity br 2.8 p 2.1 mg 1.2 la 1.0 cs 0.7 Thus, fluorine is the most electronegative element, while francium is one of the least electronegative.

Element electronegativity br 2.8 p 2.1 mg 1.2 la 1.0 cs 0.7 Thus, fluorine is the most electronegative element, while francium is one of the least electronegative. Therefore, the correct answer is d.
 Source: angelo.edu
Source: angelo.edu
In the case of carbon, bromine and fluorine, they all can gain electrons to achieve full outer shells (fluorine is in fact the most electronegative atom). A) 1 c) b) 6 d) 2 7. The alkaline earth element having the largest atomic radius is found in period:
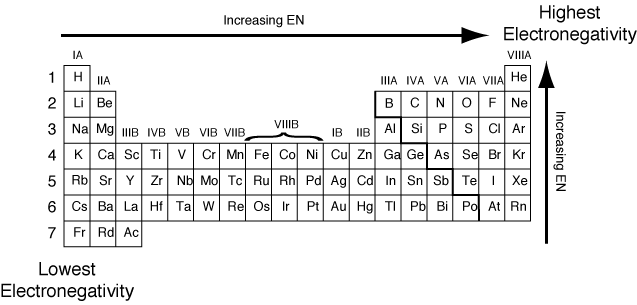 Source: socratic.org
Source: socratic.org
The lowest electronegativity of the element from the following atomic number is. Therefore, the correct answer is d. Electronegativity is the property of an element to attract electrons towards itself.
 Source: thoughtco.com
Source: thoughtco.com
Group 18 elements have no electronegativity. Based on the indicated electronegativities, arrange the following in order of increasing ionic character: The electronegativity of calcium (ca) is 1.
 Source: breakingatom.com
Source: breakingatom.com
The allen scale assigns the lowest electronegativity to cesium, with a value of 0.659. They have few valence electrons and aren’t close to having eight valence electrons The element in period 3 with the most metallic character is:
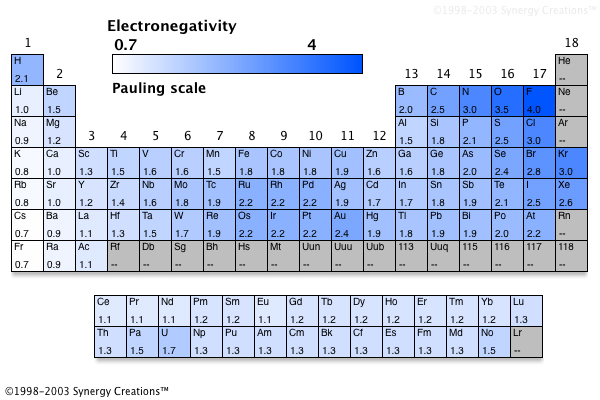 Source: socratic.org
Source: socratic.org
Which of the following elements has the lowest electronegativity? B beryllium is a metal, and metals have higher values of electronegativity than nonmetals of the same period.; Choice selenium (se) bromine (br) arsenic (as) germanium (ge) 10b) which of the following elements has the lowest electronegativity?
 Source: sciencenotes.org
Source: sciencenotes.org
What is the numerical value? Fluorineof the main group elements, fluorine has the highest electronegativity (en = 4.0) and cesium the lowest (en = 0.79). A measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound is called.
 Source: study.com
Source: study.com
The allen scale assigns the lowest electronegativity to cesium, with a value of 0.659. The electronegativity of strontium (sr) is 0.95. The electronegativity of calcium (ca) is 1.
Also Read :





